
Giuseppe Pettinato, Ph.D., is a molecular biologist with a doctorate in Embryology from the University of Catania, completed through a collaborative program with the Andalusian Center for Molecular Biology and Regenerative Medicine (CABIMER) in Seville, Spain. With over 20 years of experience in stem cell biology, organoid engineering, and translational medicine, he is currently an Instructor in Medicine at Harvard Medical School and the PI and Director of the Stem Cells and Organoids Research Engineering (SCORE) Center within the Division of Gastroenterology at Beth Israel Deaconess Medical Center.
Dr. Pettinato has developed innovative and scalable methodologies for generating human organoids from hiPSCs, including microfabricated microwell arrays that promote rapid and uniform organoid formation. His laboratory has established robust differentiation protocols for liver organoids and is extending this work to pancreas and intestinal systems. These platforms are being used to model complex human diseases, including NAFLD, NASH, ALD, HBV infection, type 1 diabetes, and inflammatory bowel diseases, providing powerful tools for mechanistic studies, drug discovery, and translational therapeutic development.
Nanoparticle-mediated delivery of alpha-1 antitrypsin reduces cytokine-induced inflammation affecting islet transplantation
Matthew Massaad1, Hamid Mostafavi Abdlumaki1, Wayne J Hawthorne2, Xuejun Wen3, Giuseppe Pettinato1.
1Medicine, BIDMC/Harvard Medical School, Boston, MA, United States; 2Surgery, Westmead Hospital, University of Sydney, Westmead, Australia; 3Chemical and Life Science Engineering, Virginia Commonwealth University, Richmond, VA, United States
Introduction: Type 1 diabetes affects millions worldwide and, despite treatment advances, many patients still face frequent, dangerous hypoglycemia due to impaired hormonal responses and hypoglycemia unawareness. Allogeneic islet transplantation can restore insulin production and prevent severe hypoglycemia in these high-risk patients, but early inflammation and oxidative stress limit graft success. TNF-α inhibitors and alpha-1 antitrypsin have shown promise in enhancing graft survival by modulating immune responses. Our study introduces a proof-of-concept strategy using A1AT-loaded, redox-responsive nanoparticles co-transplanted with islets to locally reduce inflammation, enhance graft survival, and improve engraftment efficiency while reducing the side effects of systemic immunosuppression.
Methods: Human pancreatic islets were obtained from Prodo Laboratories Inc. and cultured under standard conditions. Redox-responsive nanoparticles encapsulating alpha-1 antitrypsin (SR-A1AT-NPs) were synthesized using a dextran-based formulation and applied to the islet cultures for 24 hours. Nanoparticle uptake was confirmed via fluorescent labeling and confocal microscopy. Islets pre-treated with SR-A1AT-NPs were subsequently exposed to an in vitro inflammatory cocktail (Cytomix: TNF-α, IL-1β, and IFN- γ). Viability and mitochondrial function were assessed using G6PD and MTT assays, respectively. Flow cytometry with Annexin V and propidium iodide staining was performed to quantify live/dead and early/late apoptotic populations. Functional integrity was evaluated via glucose-stimulated insulin secretion (GSIS) assays.
Results: SR-A1AT-NPs treatment conferred significant protection to human islets under proinflammatory conditions whilst in culture for 24 hours. Flow cytometry analysis revealed a reduction in both late apoptotic and dead cell populations when treated with SR-A1AT-NPs (Fig 1). Compared to untreated controls exposed to cytokine stress, nanoparticle-treated islets demonstrated improved mitochondrial activity, reduced cytotoxicity, and enhanced viability (Fig. 2A-B). Additionally, GSIS assays showed that AAT-loaded nanoparticle treatment preserved insulin secretory function whilst under proinflammatory conditions in response to glucose stimulation (Fig. 2C).
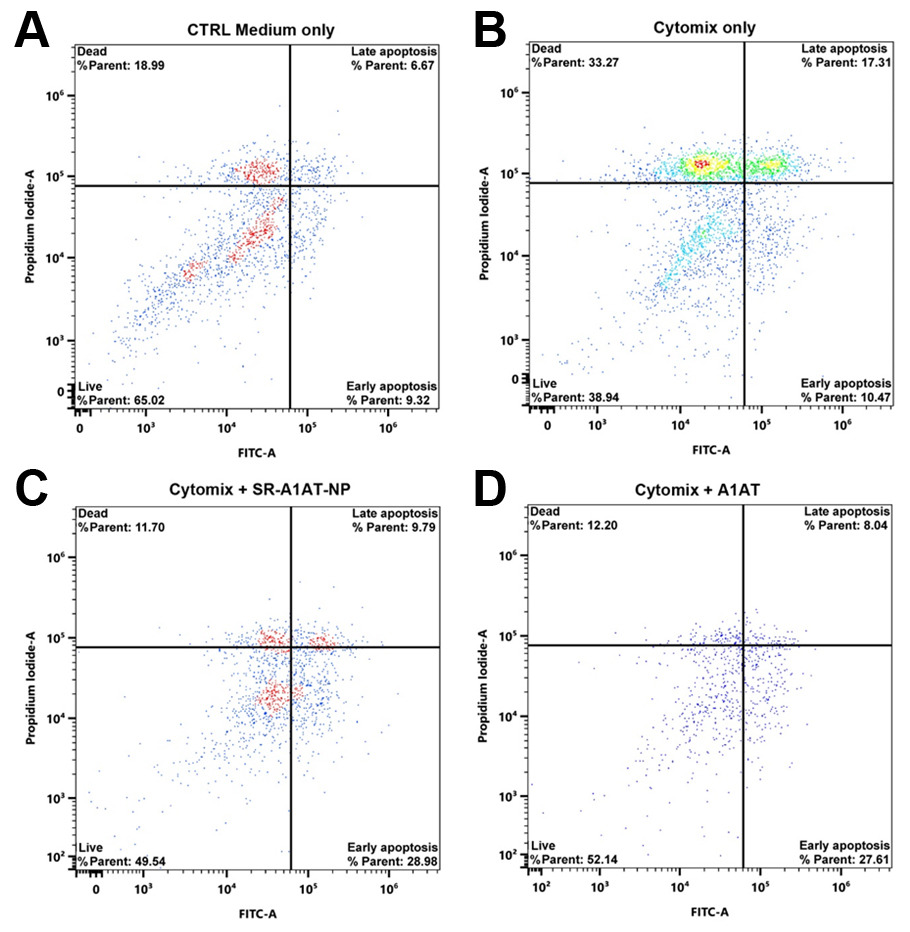
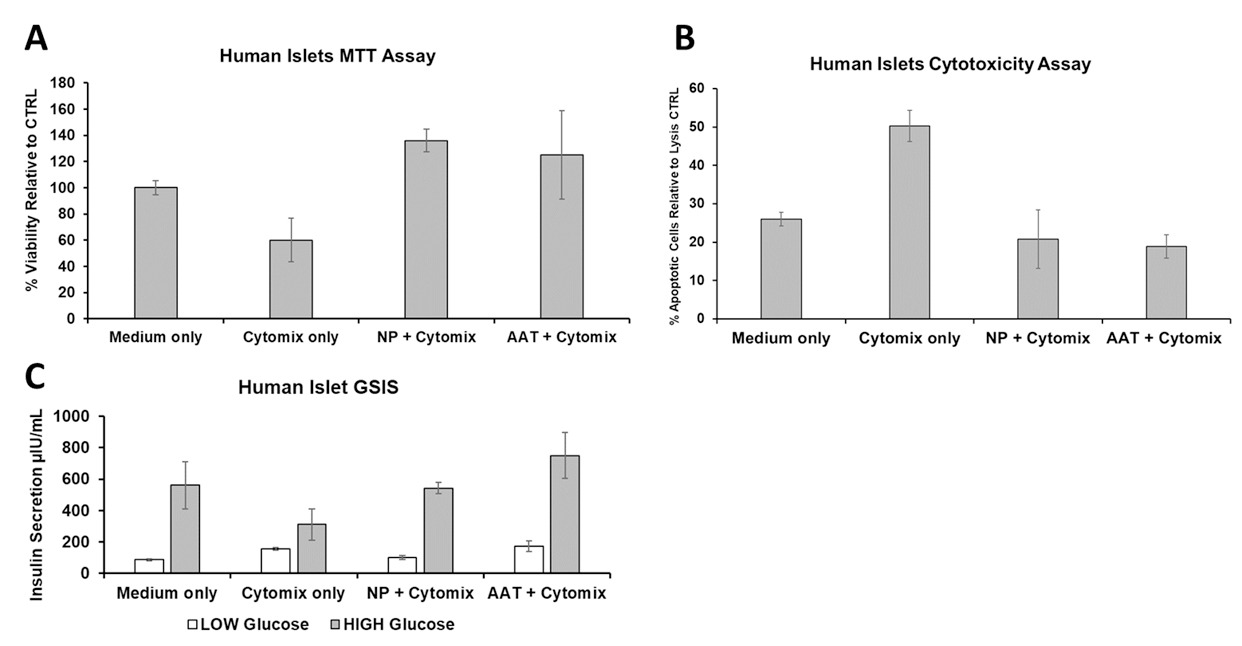
Conclusion: These findings demonstrate that SR-A1AT-NPs protect human islets from inflammatory injury while preserving metabolic function. By enabling localized delivery of an immunomodulatory agent, this strategy provides an enhanced method to reduce early inflammatory damage and offers a safer, targeted method to enhance islet graft survival and function. This approach has the potential to increase the efficiency of islet engraftment and broaden the clinical applicability of islet transplantation in T1D by reducing the instant blood mediated inflammatory reaction (IBMIR).
We thank Breakthrough T1D (formerly JDRF) for providing the funding for this project. .
[1] Human Pancreatic Islet Transplantation, Slow-Release-A1AT-Nanoparticles, Local Drug Delivery, Anti-inflammatory Treatment, Improved Engraftment.