
Giuseppe Pettinato, Ph.D., is a molecular biologist with a doctorate in Embryology from the University of Catania, completed through a collaborative program with the Andalusian Center for Molecular Biology and Regenerative Medicine (CABIMER) in Seville, Spain. With over 20 years of experience in stem cell biology, organoid engineering, and translational medicine, he is currently an Instructor in Medicine at Harvard Medical School and the PI and Director of the Stem Cells and Organoids Research Engineering (SCORE) Center within the Division of Gastroenterology at Beth Israel Deaconess Medical Center.
Dr. Pettinato has developed innovative and scalable methodologies for generating human organoids from hiPSCs, including microfabricated microwell arrays that promote rapid and uniform organoid formation. His laboratory has established robust differentiation protocols for liver organoids and is extending this work to pancreas and intestinal systems. These platforms are being used to model complex human diseases, including NAFLD, NASH, ALD, HBV infection, type 1 diabetes, and inflammatory bowel diseases, providing powerful tools for mechanistic studies, drug discovery, and translational therapeutic development.
Targeted Delivery of Alpha-1 Antitrypsin via Nanoparticles Attenuates Cytokine-Induced Inflammation In Vitro
Matthew Massaad1, Hamid Mostafavi Abdlumaki1, Xuejun Wen2, Robert A Fisher1, Giuseppe Pettinato1.
1Medicine, BIDMC/Harvard Medical School, Boston, MA, United States; 2Chemical and Life Science Engineering, Virginia Commonwealth University, Richmond, VA, United States
Introduction: End-stage liver disease is life-threatening and primarily treated by transplantation, which carries surgical risks and requires lifelong immunosuppression to prevent immune rejection. Inflammation and ischemic injury from transplantation can cause early graft damage despite immunosuppressants. To address donor shortages and mitigate immunosuppression side effects, bioengineered liver substitutes, such as hiPSC-derived liver organoids, show great promise. This study investigates the use of slow-release alpha-1 antitrypsin (A1AT)-loaded nanoparticles incorporated into liver organoids to reduce early post-transplant inflammation and support tissue repair in vitro, to enhance immediate graft function and survival.
Method: Human embryoid bodies (hEBs) were produced using our custom agarose micro-molding platform, and then directed through a hepatic lineage specification protocol to yield liver-like organoids. Nanoparticles encapsulating alpha-1 antitrypsin (A1AT) for controlled release (SR-A1AT-NP) were fabricated via a dextran-based formulation and subsequently applied to the organoid cultures. These engineered organoids were then subjected to an in vitro inflammatory challenge using a cytokine mix (TNF-α, IL-1β, and IL-6, collectively referred to as Cytomix), and their viability and cellular health were assessed via MTT and G6PD assays, providing metrics on live and apoptotic cell populations. For in vivo validation, SR-A1AT-NP-containing liver organoids were transplanted into a rat model of acute liver failure to evaluate their capacity to mitigate inflammatory damage post-transplant. Engraftment efficiency was determined by quantifying human albumin in the host serum, and the therapeutic benefit was gauged by comparing survival and liver function outcomes between treated and control groups.
Results: Liver organoids incorporating SR-A1AT-NP exhibited significantly enhanced viability upon Cytomix exposure for 24 hours in vitro, relative to their nanoparticle-free counterparts (Fig. 1). In addition, total protein content was maintained at levels comparable to untreated controls (Fig. 2). Following in vivo transplantation, SR-A1AT-NP organoids led to marked improvements in graft function, graft and recipient survival with sustained albumin production (Table. 1).
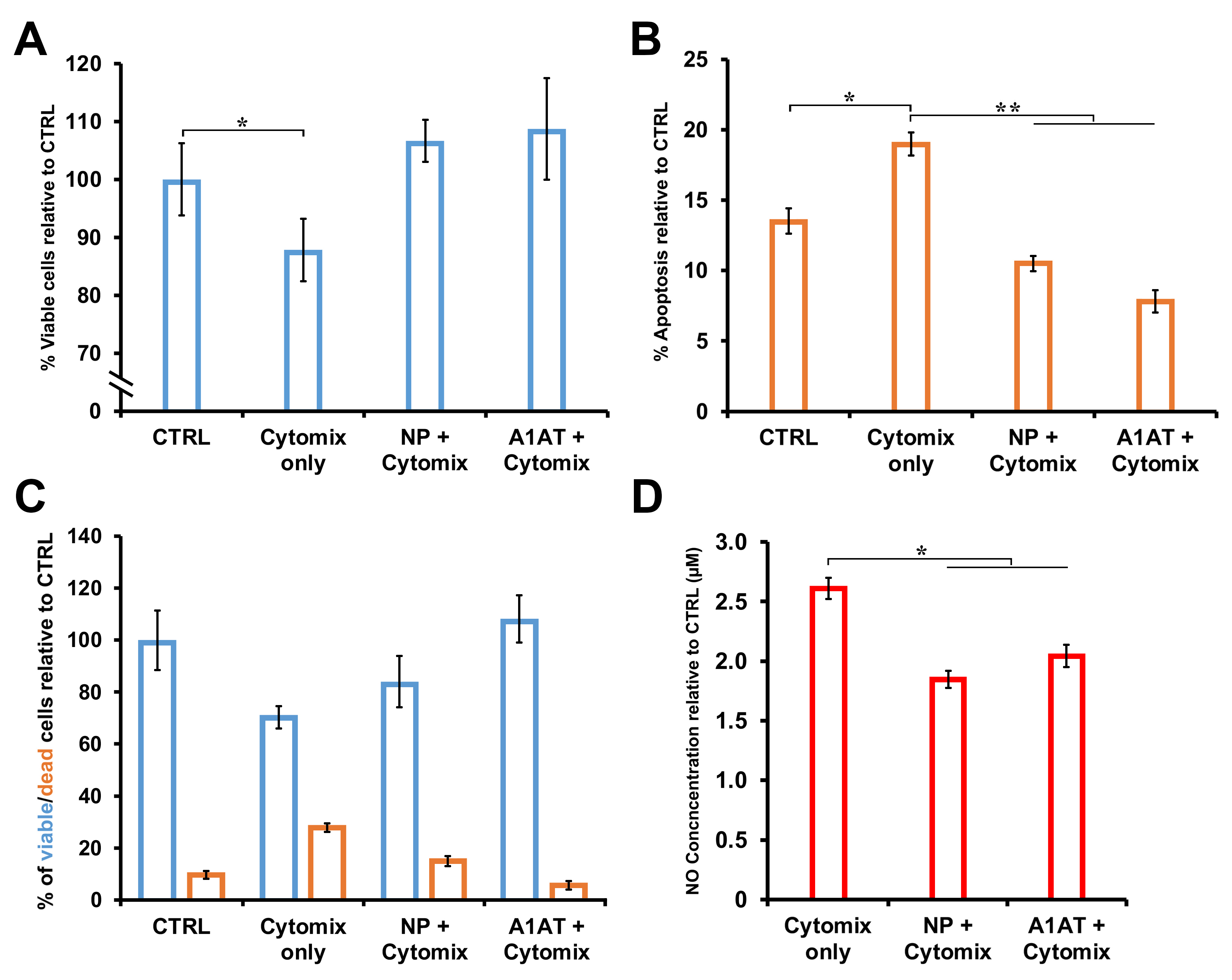
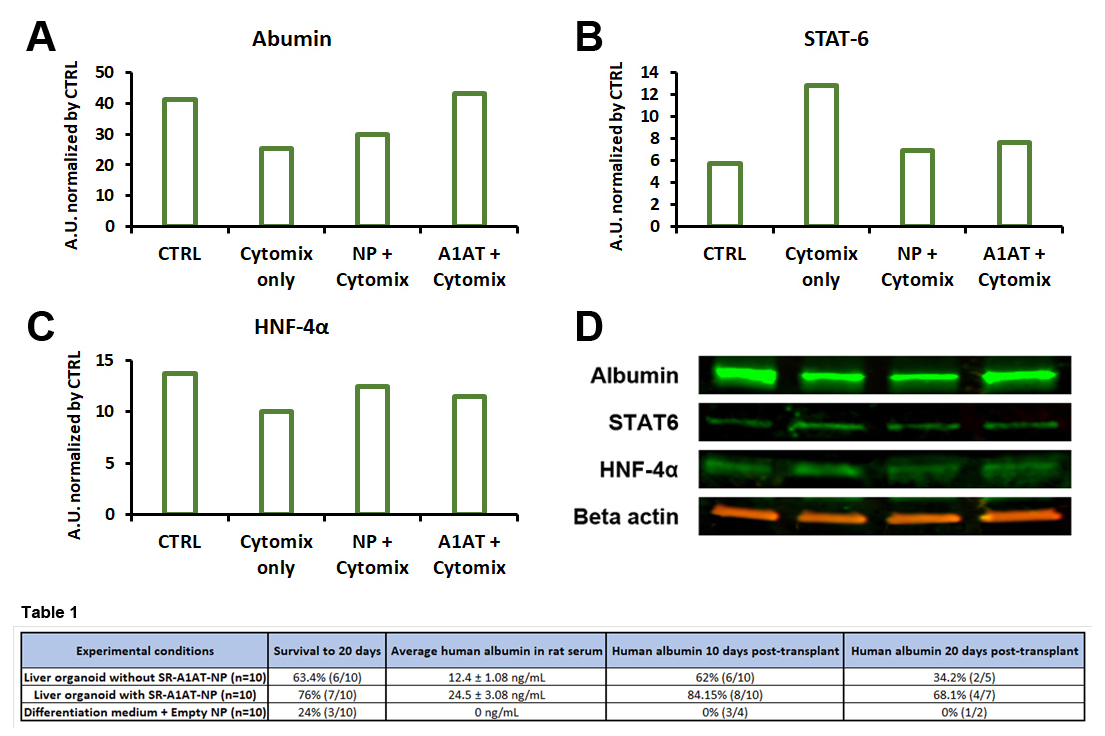
Conclusion: Liver organoids loaded with SR-A1AT-NPs exhibited a protective response against cytokine-induced damage in vitro, as evidenced by sustained overall cell viability and preserved liver function. This was confirmed through the consistent expression of key hepatic markers, including albumin and HNF-4α, in comparison to Cytomix-treated controls, along with a reduction in STAT-6 levels observed in both untreated and treated organoids.
[1] Liver organoids, Slow-release loaded nanoparticles, Alpha-1 Antitrypsin, Inflammation reduction