
Development of dissolving microneedles containing finasteride with improved permeability and bioavailability
Minseok Kang1, Ga-Young Sim1, Chul hyun Park1, Nahyun Kim1, Youn seop Kim1, Dongho Lee1, Kyungho Baek1,2, Han-Gon Choi2, Sung Giu Jin1.
1Department of Pharmaceutical Engineering, Dankook University, Cheonan-si, Korea; 2Pharmacy & Institute of Pharmaceutical Science and Technology, Hanyang University, Ansan-si, Korea
Introduction: Androgenetic alopecia (AGA) is the predominant type of hair loss and significantly affects patients’ quality of life. Finasteride (FNS) is commonly prescribed as an oral agent for AGA management, but its systemic administration may lead to adverse effects such as depression, reduced libido, and erectile dysfunction. Dissolving microneedles (DMNs) are capable of penetrating the stratum corneum and enhancing transdermal delivery efficiency in a minimally invasive manner. In this study, to reduce side effects and achieve greater efficacy, FNS was formulated as dissolving microneedles to provide a novel administration route and improved skin absorption, and its performance was evaluated.
Methods: DMNs were fabricated using a solvent casting method with a vacuum. DMNs were designed with a bilayer structure, with the first layer (the tip), which loaded FNS, prepared using various ratios of sorbitol. The base layer was formulated with PVP and PVA. The polymer ratio was selected through mechanical property testing using a Texture analyzer. The in vitro release profile was studied in distilled water and PBS pH 6.8, and skin penetration studies were assessed via Franz diffusion cells in rat skin (PBS pH 6.8, 32°C). Samples were analyzed using HPLC.
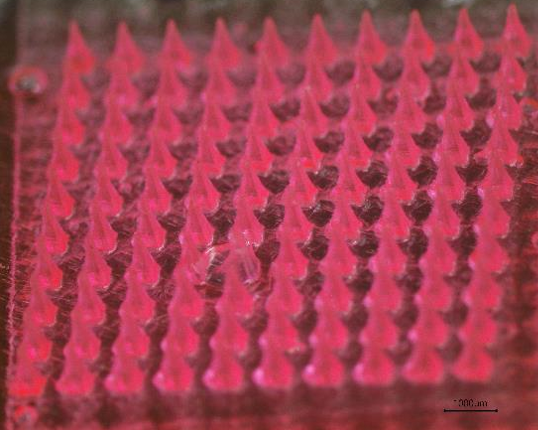
Results: DMNs exhibited a uniform morphology, as shown in Figure 1. Over 90% of DMNs penetrated 7th parafilm layers. These findings validate the microneedles' mechanical capacity to breach the stratum corneum, a critical barrier in transdermal drug delivery. In vitro release studies demonstrated that DMNs exhibited faster release rates and higher cumulative drug release percentages, compared to pure drug. Furthermore, ex vivo skin permeation studies revealed that DMNs loaded with FNS achieved higher transdermal permeation efficiency than films. This improvement is attributed to the DMNs' ability to disrupt the stratum corneum barrier, thereby facilitating efficient drug permeation into deeper skin layers. These results demonstrate DMN technology's ability to enhance topical drug delivery for AGA treatment.
Conclusion: This study designed DMNs containing FNS. The microneedles were structurally optimized to bypass the stratum corneum barrier, thereby enhancing drug permeation into deeper dermal layers for targeted therapeutic action. These findings not only support DMNs' potential for AGA treatment but also demonstrate their innovative applicability in transdermal drug delivery by overcoming skin barrier limitations. Future studies will evaluate the therapeutic efficacy for AGA through vivo evaluation of hair growth promotion.
This work was supported by a National Research Foundation of Korea (NRF) grant under the Korean government (MEST) (No. RS-2023-00208448). .
[1] Dissolving microneedle
[2] Finasteride