Liver-derived mesenchymal stem cells increase cancer cell plasticity through TGF-beta pathway activation in a hepatocellular carcinoma spheroid co-culture model
Grégory de Bodt1,2, Joachim Ravau1, Jonathan Evraerts1, Xavier Stéphenne Pr1,2, Mustapha Najimi Dr1, Etienne Sokal Pr1,2.
1PEDI laboratory, IREC, UCLouvain, Brussels, Belgium; 2Pediatric Gastro-Enterology and Hepatology unit, Cliniques Universitaires Saint-Luc, Brussels, Belgium
Background and Aims: Mesenchymal stem cells (MSCs) play a highly debated role in cancer. Potent anti- and pro-tumor properties have been shown in vitro and in vivo, depending on the tumor studied and the MSC used. Here, we investigate the interaction between liver-derived MSCs and hepatocellular carcinoma cells (HCC), with a focus on cell proliferation, invasiveness and gene expression. We postulate that their shared hepatic origin may provide unique insights into the potential implication of MSCs in carcinogenesis.
Method: We developed a 3D bi-cellular spheroid model comprised of Human Adult Liver Progenitor Cells (HALPCs), liver-derived MSCs previously described in our laboratory, and one of three human HCC cell lines (HuH7, HepG2, Hep3B). Cancer cell proliferation was studied through spheroid growth follow-up and Ki67 immunostaining using light sheet fluorescence microscopy. Cancer cell invasiveness was investigated using transwell and spheroid invasion assays. RNA sequencing was conducted on HALPCs and cancer cells from control and co-culture spheroids, separated by fluorescence-activated cell sorting.
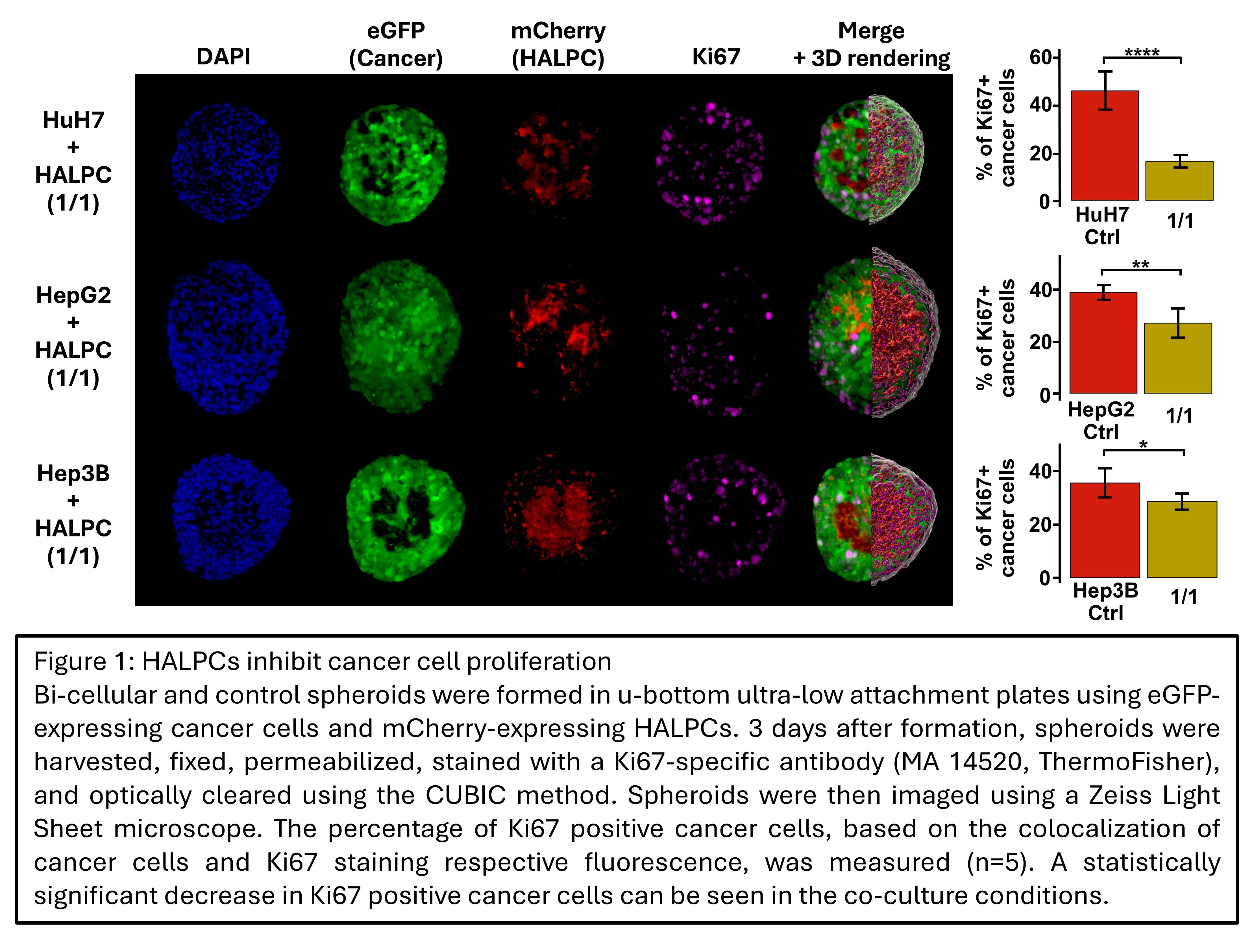
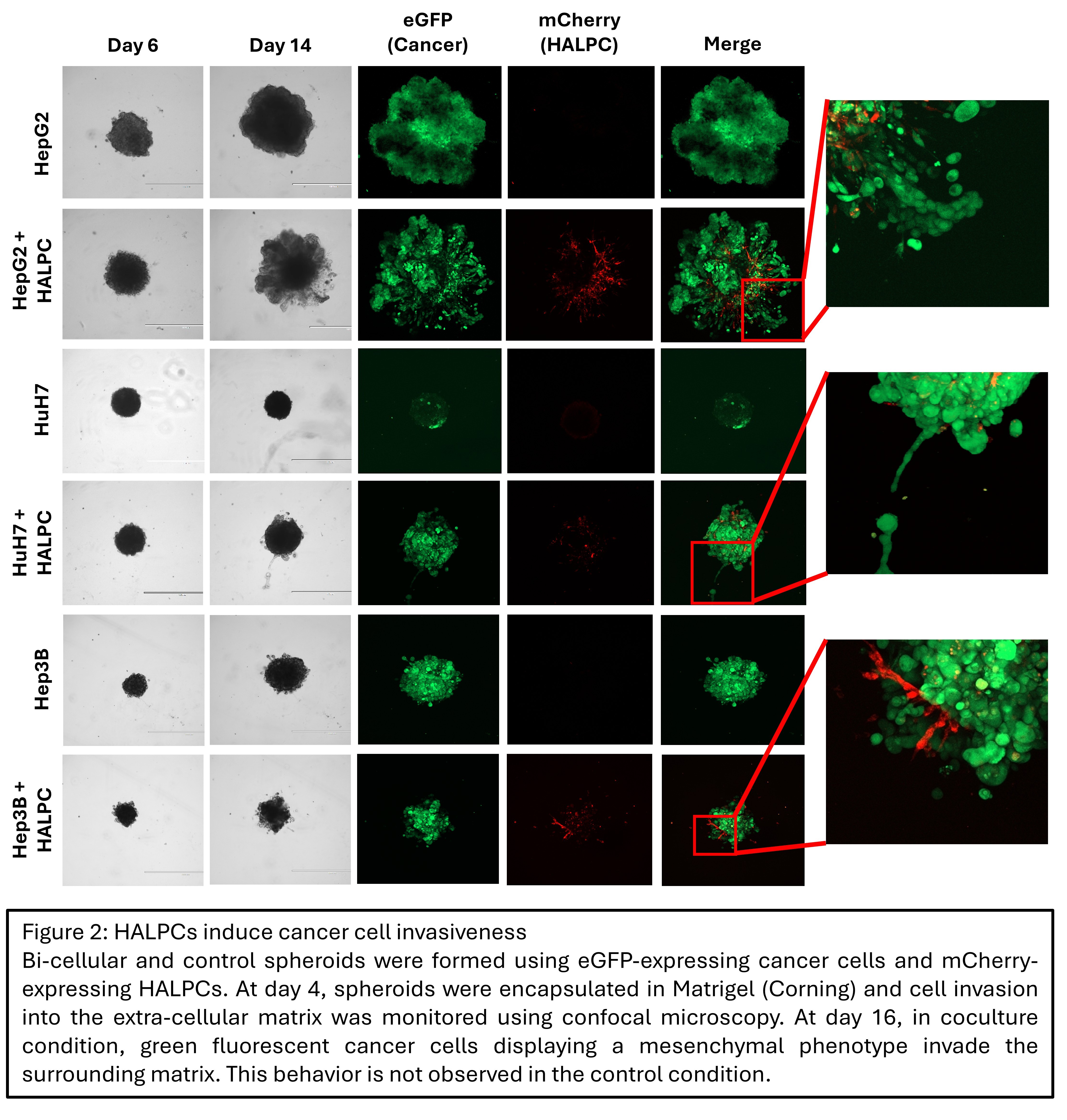
Results: HCC 3D co-culture with HALPCs resulted in a dose-dependent inhibition of spheroid growth. At 25 days post-formation, the volume of control spheroids was up to 70 times larger compared to 50:50 co-culture spheroids. This difference in spheroid size was associated with a reduction of up to 66% in Ki67-positive cells in co-culture conditions (p = 4.6x10-5) (Figure 1). Invasion studies demonstrated the induction of an invasive phenotype in the cancer cell lines when co-cultured with HALPC (Figure 2). Comprehensive transcriptomic analysis showed substantial changes in expression levels, with 2002 and 3601 up- or downregulated genes in HALPCs and cancer cells, respectively (| logFC | > 1 ; p adj. < 0.05). In co-cultured cancer cells, an increased cell plasticity was observed, with induction of a partial epithelial-mesenchymal transition and hepatocyte-to-cholangiocyte trans-differentiation. Co-cultured HALPCs showed an increase in cancer-associated fibroblast (CAF) transcriptomic signature. These changes occur simultaneously with the activation of the TGF-beta pathway at the transcriptomic level in both cell types.
Conclusion: We demonstrate that liver-derived MSCs decrease the proliferation and induce the invasiveness of several human HCC cell lines. This is associated with the induction of cancer cell plasticity, the increase in the CAF signature of HALPC, and the overall activation of the TGF-beta pathway in both cell types at the transcriptomic level. These findings support the potential role of organ-resident MSCs in HCC carcinogenesis. Ongoing work aims to confirm the transcriptomic findings at the protein level, verify the implication of TGF-beta in the acquired phenotype through pathway inhibition and validate the obtained results in vivo, using an orthotopic xenogeneic bioluminescent mouse model.
Grégory de Bodt is a Research Fellow of the Fonds de la Recherche Scientifique – FNRS.
[1] 3D culture
[2] Mesenchymal Stem Cells
[3] Hepatocellular Carcinoma
[4] Light Sheet microscopy
[5] Transcriptomics
[6] Cancer
[7] Cell therapy
[8] Liver