Metabolic reprogramming affects the stability of Tregs in inflammatory environments
Qufei Qian1, Jian Gu1, Ling Lv2.
1The First Affiliated Hospital of Nanjing Medical University, Nanjing, People's Republic of China; 2The Affiliated Hospital of Xuzhou Medical University, XuZhou, People's Republic of China
Ling Lab.
Introduction: Regulatory T cells (Tregs), as critical immunosuppressive cell subsets, play a central role in maintaining immune homeostasis. Treg cells will exhibit metabolic and functional instability in an inflammatory environment, shifting from originally inhibiting inflammation to promoting inflammatory phenotypes. Therefore, improving the stability of Treg in an inflammatory environment is of great significance for the clinical reinfusion therapy of Treg cells. Our research confirmed that Treg cells first underwent metabolic reprogramming in an inflammatory environment, and then the changes in Treg function occurred due to metabolic alterations. Subsequently, we verified the pathways and targets of metabolic changes of Treg in the inflammatory environment in the GVHD model, expecting its application in clinical treatment in the future.
Methods: The status of reinfused Treg cells was detected on the 2nd day, 4th day, 6th day, 1st week, 2nd week and 3rd week in the GVHD model. The reinfused Treg cells in the GVHD model were sorted out by flow cytometry, and CAR-Treg was designed for the found targets for reinfusion verification.
Results:We constructed a GVHD model, and transfused HLA-A2-positive Treg with or without HLA-A2-negative PBMC back into irradiated NCG mice, and sorted out the Treg cells transfused in the spleen of mice at week 1, 2, and 3. It was found that foxp3 expression and cell stability of Treg cells decreased under inflammatory environment 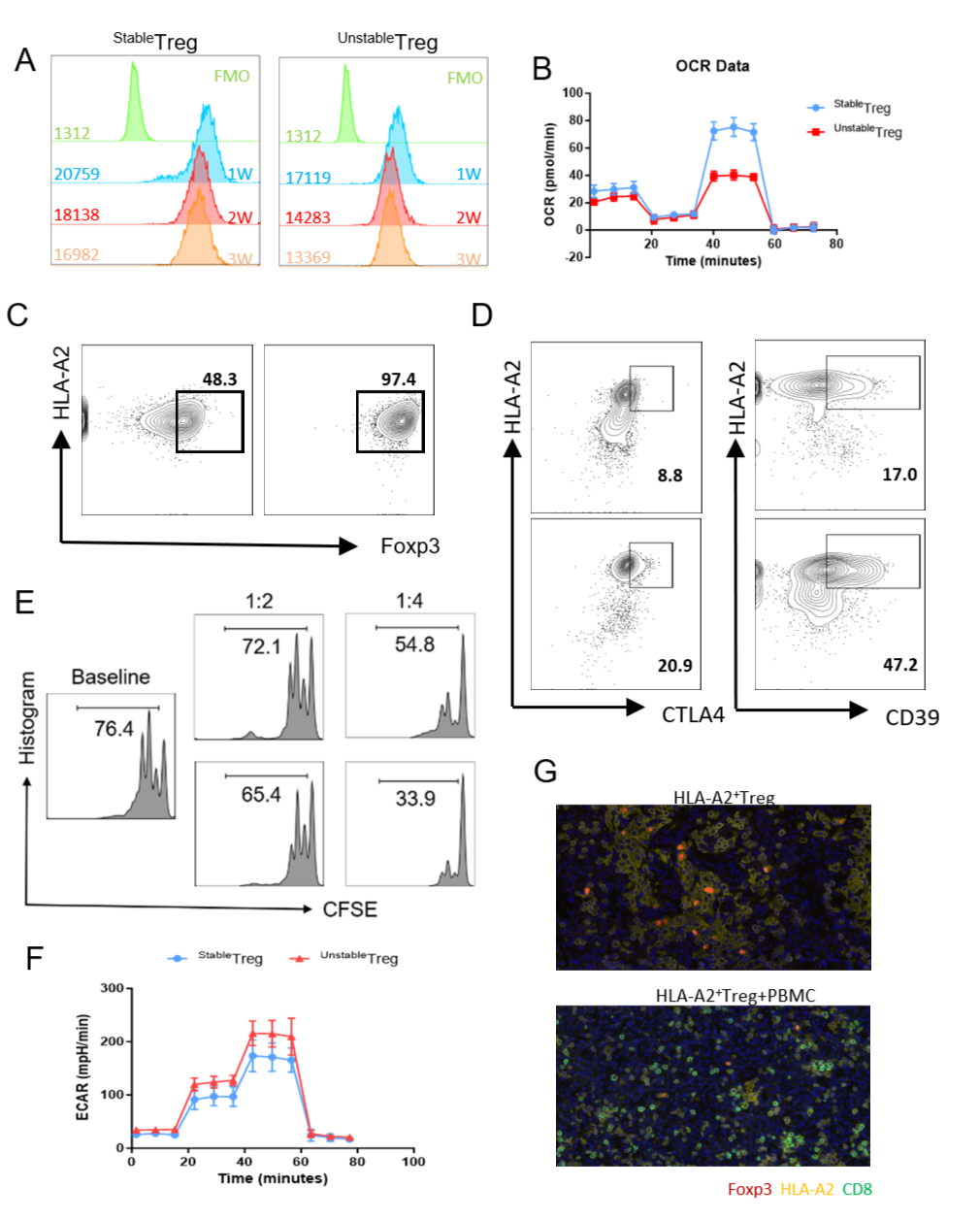 The results of single-cell sequencing showed that metabolic reprogramming occurred earlier than the emergence of functional instability, suggesting that metabolic remodeling in an inflammatory environment may be one of the reasons for the instability of Treg cells.
The results of single-cell sequencing showed that metabolic reprogramming occurred earlier than the emergence of functional instability, suggesting that metabolic remodeling in an inflammatory environment may be one of the reasons for the instability of Treg cells. 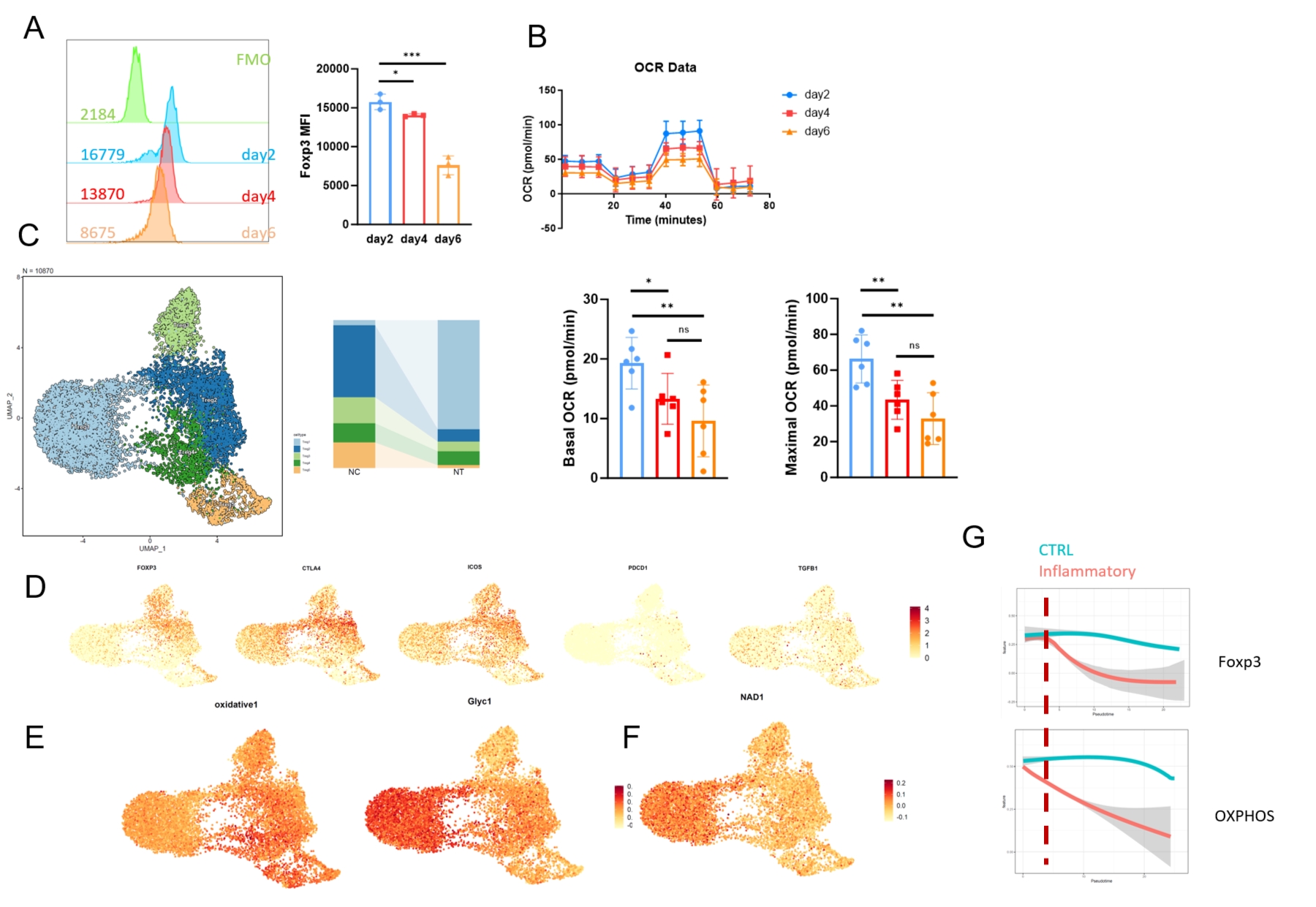 We found a significant decrease in AMPK signal in the inflammation-specific Treg subgroup. We found that AMPK agonist-treated Tregs were more effective against GVHD. We performed Ribo-seq on Treg cells selected from the spleen of GVHD mice, and found that the translation efficiency of Foxp3 decreased significantly in inflammatory environment. Finally, we constructed AMPK-CAR-Treg for reinfusion therapy and found that the reinfusion therapy effect of CAR-Treg cells was better.
We found a significant decrease in AMPK signal in the inflammation-specific Treg subgroup. We found that AMPK agonist-treated Tregs were more effective against GVHD. We performed Ribo-seq on Treg cells selected from the spleen of GVHD mice, and found that the translation efficiency of Foxp3 decreased significantly in inflammatory environment. Finally, we constructed AMPK-CAR-Treg for reinfusion therapy and found that the reinfusion therapy effect of CAR-Treg cells was better.
Conclusion: In the inflammatory environment, Treg first undergoes metabolic reprogramming and then shows the phenomenon of functional instability. The AMPK signaling pathway of Treg cells was specifically downregulated in the inflammatory environment. The decrease in oxidative phosphorylation levels in the inflammatory environment leads to a reduction in ATP production in cells and a decrease in the translation efficiency of key proteins in Treg cells. The increase in glycolysis level leads to the accumulation of cADPR and forms a complex with Foxp3, affecting its transcription. Finally, we constructed AMPK-CAR-Treg for reinfusion therapy and found that the reinfusion therapy effect of CAR-Treg cells was better.
National Natural Science Foundation of China (81971495, 82171759, 82101873). CAMS Innovation Fund for Medical Sciences (2019-I2M-5-035)...
[1] Immunosuppression
[2] Regulatory T cells
[3] Inflammatory Environment
[4] Treg instability
[5] Cell Metabolism