
Xi Huang is a clinical medicine graduate student in organ transplantation at the Second Affiliated Hospital of Hainan Medical University. His research focuses on xenotransplantation, with emphasis on immune rejection mechanisms in lung xenografts. His work primarily investigates macrophage innate immunity and biology.
Porcine macrophage sensing of human red blood cells reveals species-specific immune activation beyond glycan barriers
Xi Huang1, Shuai Jin1, Yong Wang2, Hidetaka Hara1, Lv-Ying Wu1, Jianan Zhang1, Tao Li1, Ting Yan1, Jinghui Li1, Dengke Pan2, Yi Wang1.
1The Transplantation Institute of the Second Affiliated Hospital, Hainan Medical University, Haikou, People's Republic of China; 2Chengdu Clonorgan Biotechnology Co., Ltd, Cheng du, People's Republic of China
Introduction: Despite progress in xenotransplantation using gene-edited pigs, innate immune barriers—particularly macrophage-mediated phagocytosis—remain a critical challenge, especially in liver and lung xenografts. Understanding how porcine macrophages interact with recipient human cells is essential to improving cross-species compatibility. This study investigates the phagocytic behavior of porcine alveolar macrophages toward human and glycoengineered pig red blood cells (RBCs), focusing on the influence of donor genetic background, surface glycan modifications, species-specific recognition, and downstream inflammatory responses.
Methods: RBCs from wild-type (WT) pigs, glycan knockout pigs (GTKO, GTKO/B4GalNT2KO, GTKO/CMAHKO, TKO), and humans were CFSE-labeled and incubated with porcine lung macrophages. Flow cytometry was used to quantify phagocytosis. Sialidase treatment of human RBCs was used to examine sialic acid–dependent recognition by porcine macrophages. Additionally, bulk RNA-sequencing was performed on macrophages after exposure to different RBC sources to assess transcriptional responses and inflammatory pathway activation.
Results: Porcine WT macrophages (n=9) preferentially phagocytosed human RBCs over pig RBCs (Auto, WT, GTKO; P<0.001; GTKO/B4GalNT2KO; P<0.05), while GTKO/CMAHKO and TKO RBCs were phagocytosed at levels comparable to human RBCs. Uptake of GTKO/CMAHKO RBCs was significantly greater than Auto (P<0.05), WT (P<0.05), and GTKO (P<0.01) RBCs. Sialidase (SA) treatment reduced both rosette formation and phagocytosis of human RBCs (SA[-]: 40.0% vs. SA[+]: 25.1%; P<0.05), implicating sialic acid–dependent mechanisms.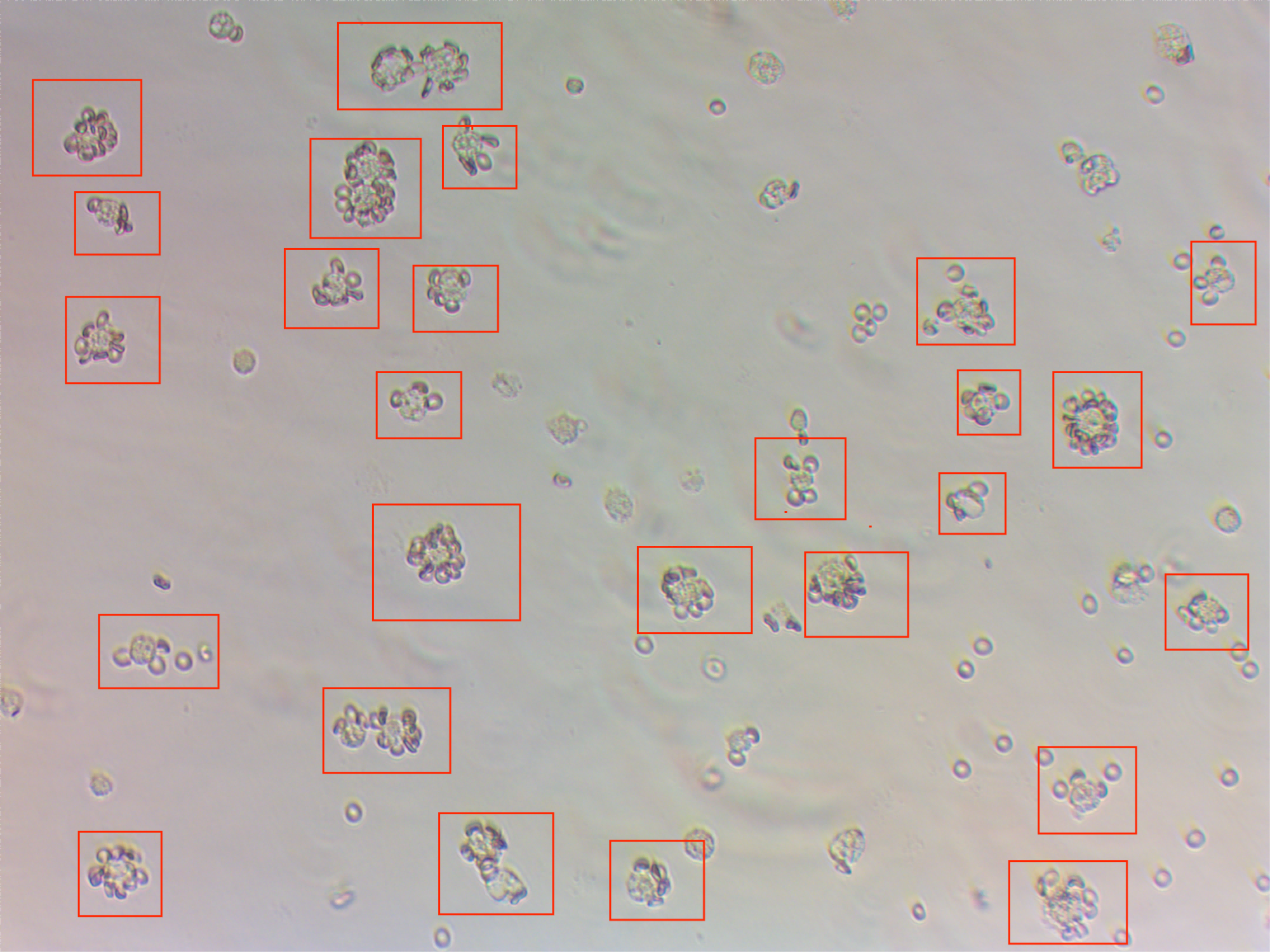
 RNA-seq analysis revealed minimal transcriptomic activation in macrophages exposed to pig RBCs, even with glycan modifications. In contrast, human RBCs triggered robust upregulation of pro-inflammatory genes (e.g., IL-17A, DKK4, MISP) and activation of key pathways such as IL-17 signaling, complement, and Th17 differentiation, suggesting the presence of glycan-independent, human-specific immune recognition.
RNA-seq analysis revealed minimal transcriptomic activation in macrophages exposed to pig RBCs, even with glycan modifications. In contrast, human RBCs triggered robust upregulation of pro-inflammatory genes (e.g., IL-17A, DKK4, MISP) and activation of key pathways such as IL-17 signaling, complement, and Th17 differentiation, suggesting the presence of glycan-independent, human-specific immune recognition.
Conclusion: Porcine macrophages exhibit preferential uptake and distinct transcriptomic responses to human RBCs, independent of glycan compatibility. These findings suggest that species-specific cellular cues beyond glycosylation—potentially involving CD47–SIRPα signaling or membrane protein disparities—contribute to innate xenogeneic immune recognition. These findings have implications not only for improving immune regulation in liver and lung xenografts, but also for the success of xenogeneic organ regeneration strategies, such as blastocyst complementation, where host macrophages may limit the survival of human-derived cells. These insights underscore the need to develop immune-modulatory strategies that address not only glycan incompatibility but also species-intrinsic innate sensing mechanisms.
This study was supported in part by the National Key Research and Development Program (2023YFC3404304: Y.W., 2024YFC3406800: H.J.), National Natural Science Foundation of China (82260154: Y.W., 82460153: H.J., 82400891: T.L.), Hainan Provincial Science and Technology Talent Innovation Project (Category B) (KJRC2023B08: Y.W.), and the Academic Enhancement Support Program of Hainan Medical University (XSTS2025029: H.H., XSTS2025161: T.L). H.H. is also supported by the Hainan Provincial High-Level Foreign Experts Recruitment Program (G20250218019E)...
[1] Xenotransplantation
[2] Macrophage phagocytosis
[3] Immune recognition
[4] Inflammation
[5] Glycan knockout