
Hannah Hagen is a rising scholar entering her senior year of the Bachelor of Science in Molecular and Cellular Biology with a minor in Biochemistry at the University of Arizona. She previously earned her Associate of Science degree with High Honors from Pima Community College in 2024.
Driven by a strong interest in medical innovation, Hannah is conducting research in cell pharmacy devices at the Institute for Cellular Transplantation for her honors thesis. Her prior research experience includes a National Science Foundation–funded Research Experience for Undergraduates (REU) at the University of Wisconsin, where she worked with Sean Scoville in the Molecular Ecology Lab. There, she explored RNA interference in synergy with insecticides to combat resistance in the Colorado potato beetle, work she later presented with support from a travel scholarship at the SACNAS 2024 NDiSTEM conference in Phoenix, Arizona.
Hannah is on track to graduate with her bachelor’s degree in May 2025 and plans to pursue a Master’s in Molecular and Cellular Biology. She intends to use the master’s program as a foundation for entering an MD–PhD, where she hopes to further her research and help bridge the gap between medicine and science.
Oxygen delivery to cells within implanted immunoisolating encapsulation devices increases the cell slab thickness and thus cell number per unit of surface area
Hannah Hagen1, Amy C Kelly1, Delaney A Drew1, Trisha M Fabijanic1, Carola G Davila1, Kelly M Pasowisty1, Jennifer P Kitzmann1, Thomas Loudovaris1, Charles W Putnam1, Robert C Johnson2, Klearchos K Papas1,2.
1Department of Surgery, University of Arizona, Tucson, AZ, United States; 2Procyon Technologies LLC, Tucson, AZ, United States
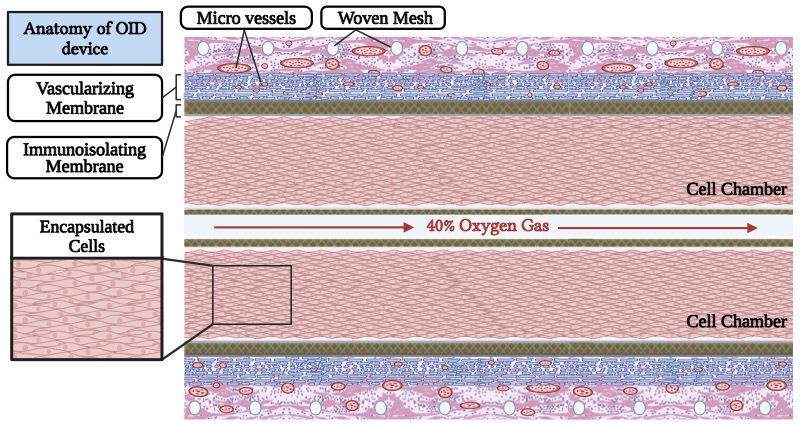
Introduction: Immunoisolating encapsulation devices when implanted subcutaneously in animal models have been shown to support allogeneic cell survival without the need for immunosuppression. However, the viability and function of cells within these devices depends upon the ambient partial pressure of oxygen (PO2) in the device. As cells are more densely loaded into smaller devices to reduce the surgical footprint, cell death increases as PO2 decreases, leaving only a thin slab of surviving cells. Here, we demonstrate that providing supplemental oxygen (O2) gas to the device increases cell slab thickness and cell number per unit surface area by over 2-fold compared to devices not provided with supplemental O2.
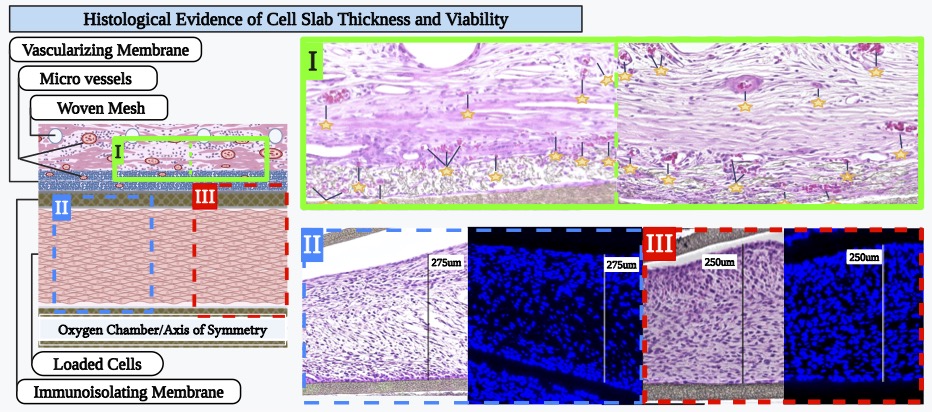
Methods: The Oxygen-enabled Immunoisolation Device (OID) has two cell chambers surrounding a lumen to which O2 gas is delivered to then diffuse to the transplanted cells. OIDs were loaded with Rat2 cells at low density (5 million cells/cm2 of device footprint area) and implanted in a dorsal subcutaneous pocket of male Ctrl: NIH-Foxn1rnu nude rats (n=5), leaving an exit site for the skin-traversing catheter which supplies O2 to the OID. OIDs were either supplied with 40% O2 gas (OID + O2) or not (OID – O2). Previous studies demonstrated that enhanced 40% O2 does not impair rat fibroblast (Rat2) viability and growth. The devices were explanted after 14 days, formalin-fixed and paraffin-embedded. Serial sections (5um) were cut from representative depths across the device and stained by H&E and DAPI for nuclei. Each section was analyzed for cell slab thickness (μm) and number of nuclei within the cell chamber (normalized to slab length and cells per cm of device). Slab dimensions and cell number per device were calculated and differences between the two groups tested (two-way ANOVA) for statistical significance. Animal subjects remained healthy and gained weight during the study. There were no interruptions of oxygen delivery to the implanted devices.
Results: O2 delivery to encapsulated devices yielded histologically evident improvements in cell health, with increased cell slab thickness by nearly three-fold (2.89) and number of nuclei by over two-fold (2.28; P<0.01 by t-test).
Conclusion: Continuous supplemental delivery of 40% O2 to subcutaneously implanted OIDs significantly improves Rat2 cell survival and health, increasing both the thickness of viable cell slabs and overall number of living cells. These findings highlight the critical role of O2 availability in supporting cell encapsulation therapies. By mitigating oxygen limitations, supplemental O₂ enables support of higher cell densities, thereby allowing for a reduced overall device footprint. The beneficial effects observed with supplemental oxygen are likely applicable to many therapeutic cell types and other encapsulation strategies, especially in high density cell-packed devices.
JDRF 3-SRA-2023-1437-M-B..
[1] Immunoisolation
[2] Cell transplant
[3] Encapsulation device
[4] Oxygen supplementation