
Dr. Giacomo Lanzoni, Ph.D.
Assistant Professor, Department of Biochemistry & Molecular Biology
Diabetes Research Institute, University of Miami Miller School of Medicine
Dr. Giacomo Lanzoni is a leading investigator focused on developing a functional cure for Type 1 Diabetes by protecting, replacing, and regenerating insulin-producing cells. His work harnesses immune modulation with mesenchymal stem cells (MSCs) and their extracellular vesicles to suppress deleterious immune responses and improve islet cell transplant outcomes. His therapeutic approach has demonstrated clinical efficacy in COVID-19 Acute Respiratory Distress Syndrome, a condition characterized by extremely elevated levels of inflammation and immune dysregulation. He is advancing localized immune protection strategies with pancreatic progenitors and stem-cell–derived islets to enable islet cell replacement at scale.
Trained at the University of Bologna (Ph.D. in Stem Cell Biotechnology), Dr. Lanzoni has published extensively, holds multiple patents, and earned international recognition. With over 17 years of translational experience bridging the laboratory and the clinic, his mission is to deliver innovative cell- and immune-based therapies that can transform the lives of people with diabetes and autoimmune conditions.
Exosomes derived from Umbilical Cord Mesenchymal Stem Cells (UC-MSCs) deliver immunomodulatory miRNA cargos and protect islet beta cells from immune-mediated injury
Arthur Rech Tondin1,2, Eric LoRusso1, Oliver Umland1, Rudy U Rodriguez1, Mayur Doke1, Rosa Hernandez1, Gianna Sweeting1,2, Max G Kanuk1,2, Joshua Menack1,2, Xiumin Xu1, Armando Mendez1,4, Juan Domínguez-Bendala1,6, Dimitrios Kouroupis1,5, Camillo Ricordi1,6,7,8, Giacomo Lanzoni1,2,3,8.
1Diabetes Research Institute, University of Miami, Miami, FL, United States; 2Department of Biochemistry and Molecular Biology, University of Miami, Miami, FL, United States; 3Dr. John T. Macdonald Foundation Biomedical Nanotechnology Institute (BioNIUM), University of Miami, Miami, FL, United States; 4Department of Medicine, Division of Endocrinology, Diabetes and Metabolism, University of Miami, Miami, FL, United States; 5Department of Orthopaedics, University of Miami, Miami, FL, United States; 6Department of Surgery, University of Miami Miller School of Medicine, Miami, FL, United States; 7Division of Cellular Transplantation, Department of Surgery, University of Miami Miller School of Medicine, Miami, FL, United States; 8Cell Transplant Center, Diabetes Research Institute, University of Miami, Miami, FL, United States
Background: The transplantation of pancreatic islets is a promising strategy to restore insulin production in patients with Type 1 Diabetes (T1D). However, immune rejection of transplanted islets remains a major challenge, necessitating chronic systemic immunosuppression, which has significant risks. Mesenchymal Stem Cells (MSCs) and their extracellular vesicles (EVs), including exosomes, possess immunomodulatory properties. MSC-derived exosomes and EVs could be harnessed to inhibit immune-mediated damage and protect insulin-producing islet β-cells.
Aim: This study aims to evaluate the effects of human Umbilical Cord-derived MSC (UC-MSC) exosomes and EVs on human pancreatic islets and immune cells, with a focus on their ability to suppress inflammation and protect β-cells from a T cell-mediated immune attack.
Methods: Exosomes and EVs were generated by culturing clinical-grade UC-MSCs in a cGMP-grade medium prepared at the Diabetes Research Institute's cGMP facility, following a standardized culture strategy. Exosomes and EVs were concentrated via Tangential Flow Filtration and characterized. Their effects were tested in cultures with activated human T cells, macrophages, islets, and in a human islet immune attack model. The effect of exosome treatment was assessed using flow cytometry for analysis of T cells and regulatory T cells (Tregs), gene expression profiling for macrophage polarization, and glucose-stimulated insulin release (GSIR) assays to evaluate β-cell function.
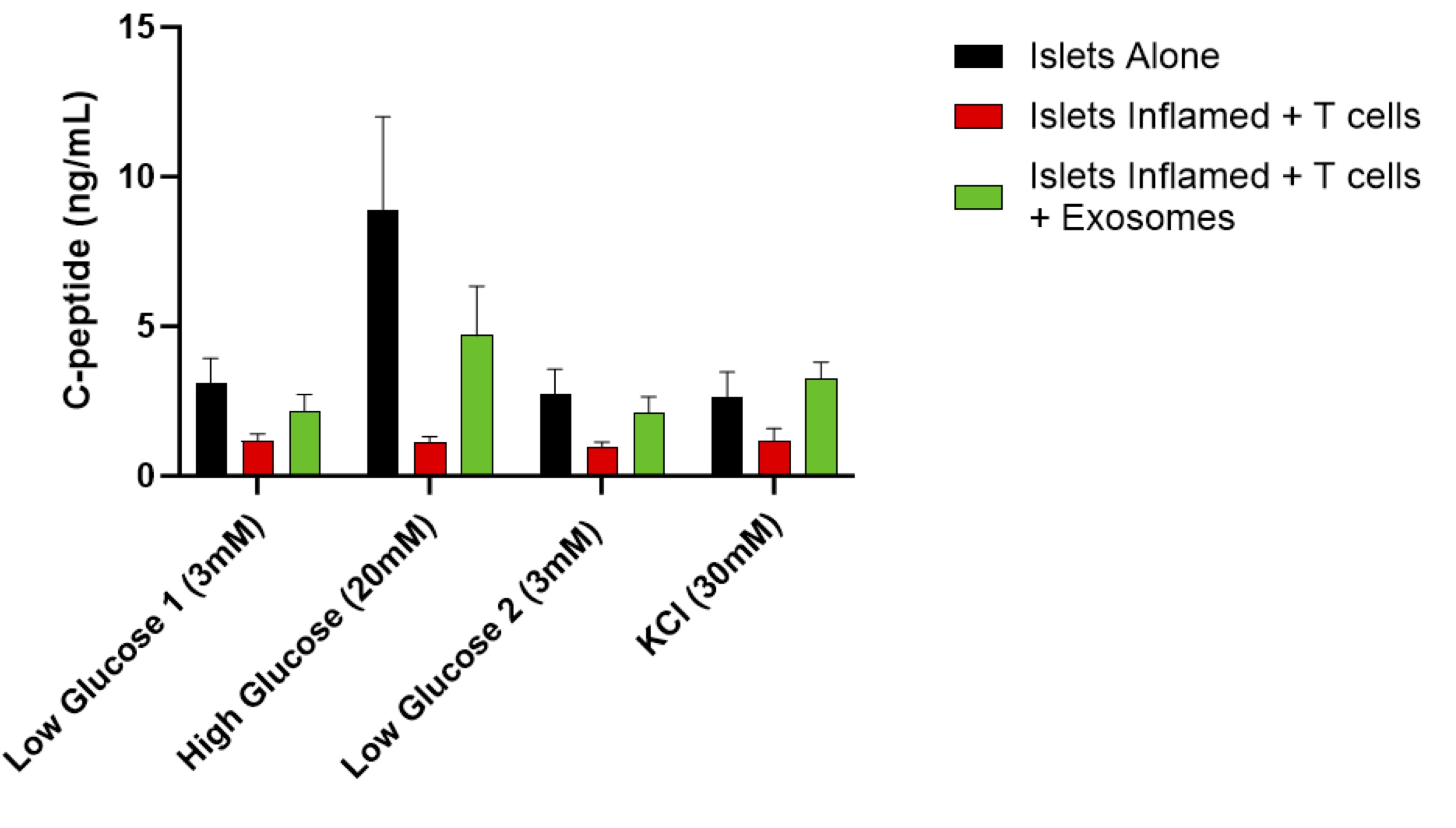
Results: UC-MSC-derived exosomes suppressed islet inflammation, induced exhaustion of cytotoxic CD8+ T cells, and promoted the expansion of Tregs and M2-like macrophages, leading to an immunoregulatory environment favoring islet survival. Exosome treatment preserved β-cell insulin secretion under inflammatory conditions and counteracted the deleterious effects of activated T cells on β-cells, maintaining β-cell function. These findings suggest that UC-MSC-derived exosomes and EVs can inhibit islet inflammation, modulate immune attacks, and protect β-cell function. Mechanistically, these effects may be partly due to the miRNA cargo of UC-MSC-derived exosomes, which regulate key inflammatory and immune signaling pathways. The miRNAs identified in EVs are associated with inhibition of pro-inflammatory cytokines, promotion of Treg stability, and polarization of macrophages toward anti-inflammatory M2 phenotype. Thus, the miRNA cargo of UC-MSC exosomes represents a plausible mechanism underlying the observed immunomodulatory and β-cell protective effects.
Conclusions: This study indicates that UC-MSC-derived exosomes and EVs exert potent immunomodulatory effects, reducing islet inflammation and preserving β-cell function. These findings support the potential of exosomes and EVs as an adjuvant strategy for islet cell transplantation, with the goal of reducing immune rejection and minimizing the need for systemic immunosuppression in islet transplantation for T1D.
Diabetes Research Institute Foundation. The Cure Alliance.
[1] Immunomodulation
[2] pancreatic islets
[3] islet cells
[4] beta cells
[5] diabetes
[6] exosomes
[7] extracellular vesicles
[8] MSC
[9] mesenchymal
[10] Mesenchymal Stem Cells