Generating amyloid resistant stem cell-derived beta cells to improve islet transplant outcomes in type 1 diabetes
Saumadritaa Kar1,2, Paul C. Orban1,4, Shugo Sasaki1,3,4, Derek Dai1,4, Galina Soukhatcheva1,4, Francis C. Lynn1,3,4, Bruce C. Verchere1,2,4,5.
1Childhood Diseases, BC Children's Hospital and Research Institute, Vancouver, BC, Canada; 2Department of Pathology and Laboratory Medicine, Faculty of Medicine, University of British Columbia, Vancouver, BC, Canada; 3Department of Cellular and Physiological Sciences, Faculty of Medicine, University of British Columbia , Vancouver, BC, Canada; 4Department of Surgery, Faculty of Medicine, University of British Columbia, Vancouver, BC, Canada; 5Centre for Molecular Medicine and Therapeutics, University of British Columbia, Vancouver, BC, Canada
Background: Transplanting insulin-producing human embryonic stem cell-derived β cells (SC-β) is a promising alternative to islet transplantation for people living with T1D. SC-β cells may obviate the need for organ donors, although their limited long-term survival and function following transplantation remains a concern.
Aggregation of islet amyloid polypeptide (IAPP), a hormone co-secreted with insulin from β cells, and the major component of cytotoxic islet amyloid in type 2 diabetes, has been implicated in islet transplant failure. The amino acid sequence in human IAPP is prone to forming β-sheets and fibrils, that contribute to islet inflammation and β-cell death. Pramlintide is a non-aggregating, non-toxic human IAPP analogue containing proline substitutions in the amyloidogenic core that is used clinically as an adjunct therapy in T1D. We hypothesize that genetically engineered SC-β cells expressing a non-amyloidogenic form of IAPP, such as pramlintide, will lead to human β-cell sources with improved survival and function following transplantation in T1D.
Methods: SC lines, CRISPR modified and GFP tagged to produce a pramlintide analogue, along with wild-type IAPP (WT) SCs were differentiated to β-cells suitable for transplant. Glucose-stimulated insulin and IAPP secretion in SC-β cells and plasma from transplanted animals were measured with a sensitive human C-peptide ELISA and in-house IAPP1-37 ELISAs respectively. Diabetic immunodeficient mice were transplanted with 75-100 sorted SC-β cell clusters in the anterior chamber of the eye (ACE). Mice fed a chow diet (n=6), or 45% high fat diet (HFD) (n=15) were assessed for blood glucose, body weight, glucose tolerance and insulin secretion with fast-refeeding.
Results: Glucose-stimulated insulin secretion suggested maturation of pramlintide and WT SC-β cells when cultured long-term. IAPP gene expression and ELISA confirmed that the mutant SC-β cells produce and secrete pramlintide. Diabetic immunodeficient mice (NSG or SCID-Beige) transplanted with WT or pramlintide SC-β cells in the ACE secrete human C-peptide in response to fast-refeed tests at 5 and 10-weeks post-transplant. Immunostained graft sections harvested 90 days post-transplant revealed abundant SC-β cells co-expressing insulin and IAPP, suggesting in vivo maturation. WT SC-β cell graft-bearing mice on HFD developed islet amyloid, while pramlintide SC-β cell grafts exhibited little or no amyloid. 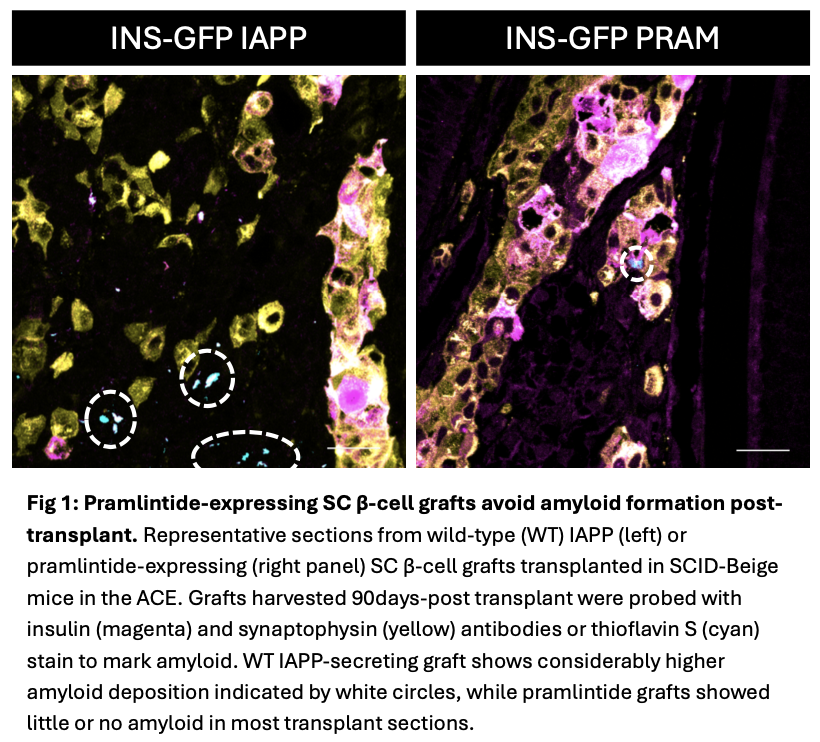
Conclusions: Our data suggest pramlintide expression does not adversely impact maturation and function of SC-β cells and may limit amyloid deposition following transplantation in the eye. Future studies will evaluate the impact of encapsulated pramlintide-expressing SC-β cell transplants in high-fat diet fed immune-deficient and immune-competent diabetic mice.
Stem Cell Network. BC Children’s Hospital Research Institute Doctoral Studentship. Canadian Islet Research and Training Network (CIRTN-R2FIC) Doctoral Trainee Award.
[1] Allograft Survival
[2] Islet Transplantation
[3] Islet Amyloid Polypeptide
[4] Type 1 Diabetes
[5] Stem Cell-derived Beta Cells