
Fei Liu, Ph.D., is an Assistant Research Fellow at Sichuan Provincial Orthopedic Hospital. His research focuses on elucidating the molecular mechanisms underlying degenerative diseases and developing novel therapeutic strategies. Dr. Liu aims to bridge fundamental discoveries with clinical translation to advance the prevention and treatment of age-related disorders. He has served as principal investigator on projects supported by the National Natural Science Foundation of China and the Sichuan Provincial Science and Technology Department. Dr. Liu has published first-author articles in high-impact journals, including Journal of Advanced Research, Redox Biology and Pharmacological Research.
Chit1 drives macrophage immune dysfunction in aging-associated kidney injury
Fei Liu1,2, Longhui Yuan1, Jingchao Yang1, Yujia Yuan1.
1National Health Commission (NHC) Key Laboratory of Transplant Engineering and Immunology, West China Hospital of Sichuan University, Chendu, People's Republic of China; 2Public Laboratory Center, Sichuan Provincial Orthopedic Hospital, Chendu, People's Republic of China
Introduction: Aging is marked by heightened vulnerability to infections and a predisposition to chronic inflammatory disorders, including chronic kidney disease, neurodegeneration, and atherosclerosis. These immunological alterations are increasingly recognized as key contributors to the pathogenesis of age-associated diseases. However, whether aging impairs immune cell-mediated regulation of inflammation and maladaptive repair after kidney injury remains unresolved.
Methods: Immune cell populations were profiled by flow cytometry. The intrinsic immunoregulatory capacity of macrophages was evaluated using RT-qPCR and fluorescent bead phagocytosis assays. Macrophage depletion in vivo was achieved with clodronate liposomes. To dissect the functional role of Chit1, we employed adoptive macrophage transfer, small interfering RNA (siRNA)-mediated gene silencing, and transcriptomic sequencing.
Results: In our study, peritoneal macrophages from aged mice exhibited a bias toward M1 polarization, along with diminished phagocytic capacity and persistent low-grade inflammation. Aging also amplified renal inflammation following acute kidney injury (AKI), marked by shifts in kidney immune cell composition, with pronounced alterations in macrophage subsets. These changes mirrored age-associated defects in the innate immune function of peritoneal macrophages, including impaired M1/M2 polarization dynamics (Fig. 1). Notably, in vivo depletion of kidney-resident macrophages in aged mice attenuated renal inflammation, mitigated tissue injury, and restored mitochondrial function. In contrast, adoptive transfer of aged peritoneal macrophages into young AKI mice exacerbated kidney damage and inflammatory responses.
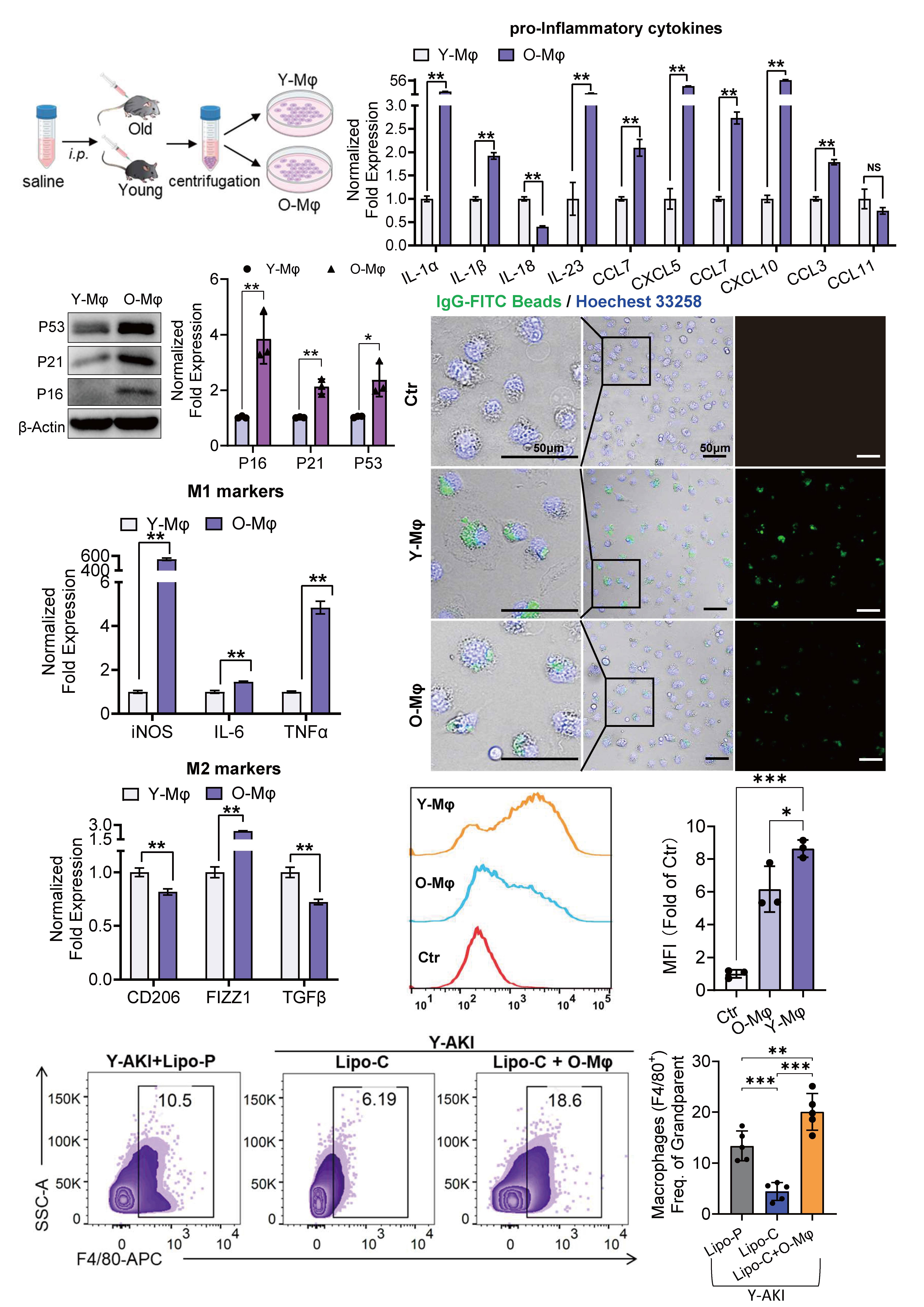
RNA sequencing revealed that the reduced immune responsiveness of aged macrophages was associated with marked upregulation of the lysosome-localized gene Chitotriosidase (Chit1). Silencing Chit1 restored immune competence in aged macrophages and alleviated mitochondrial dysfunction in cisplatin-injured renal tubular epithelial cells (TECs) via the autophagy-lysosome pathway. These findings identify Chit1 as a key regulator of macrophage immune function in young mice. Transcriptomic profiling of Chit1-silenced macrophages further revealed significant enrichment of mitochondrial-associated pathways, with BDNF showing the most robust upregulation (Fig. 2). Protein-protein docking analysis predicted a potential interaction interface between Chit1 and BDNF, suggesting that Chit1 deficiency enhances macrophage immune function, at least in part, through direct modulation of mitophagy via the BDNF/TrkB axis.
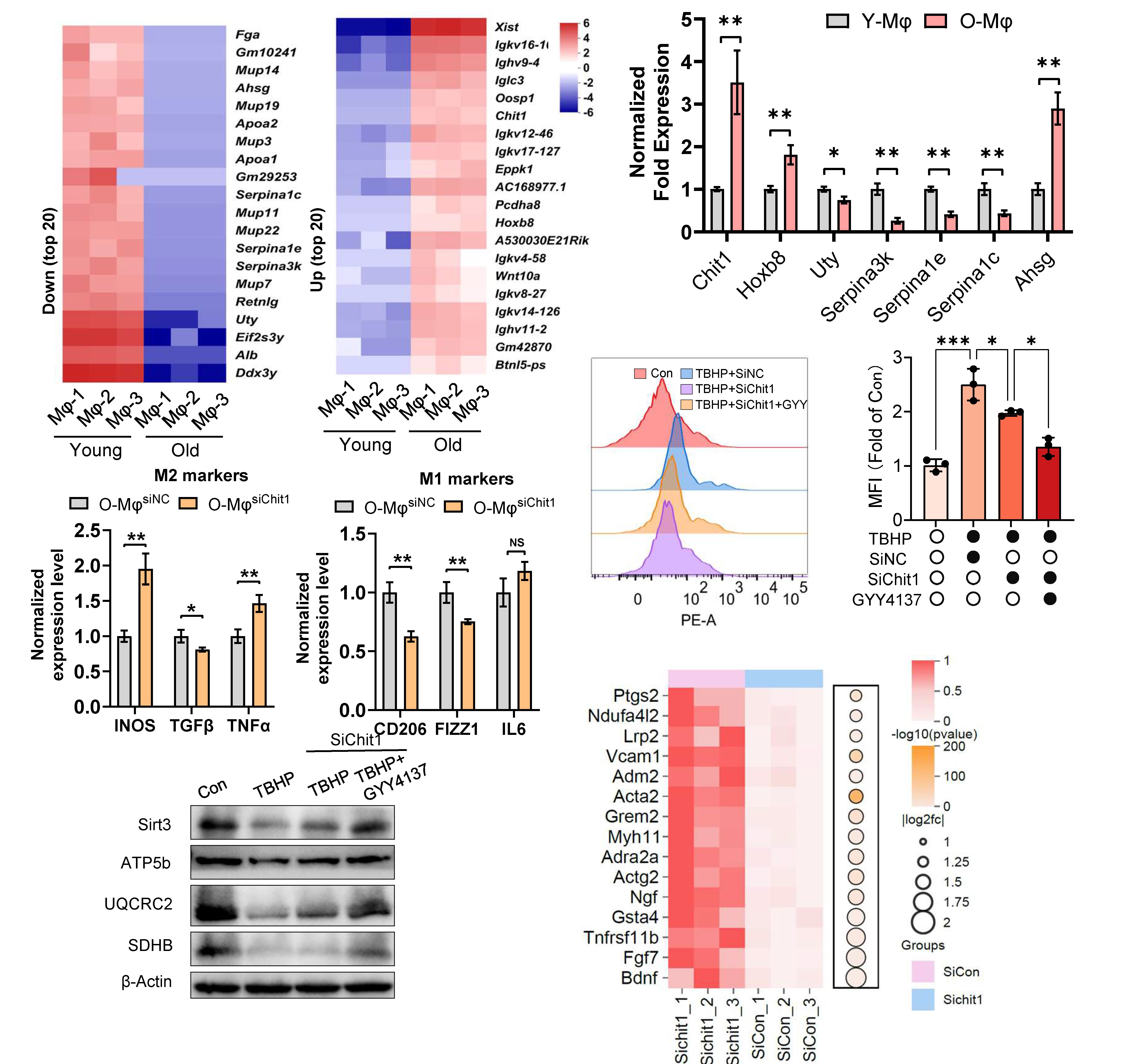
Conclusion: These findings suggest that Chit1 upregulation in aged macrophages disrupts mitophagy, and that its targeted inhibition may underlie the age-related decline in protective immune responses.
The National Natural Science Foundation of China (NSFC No.8202756). The Project of Sichuan Provincial Department of Science and Technology (No.2025ZNSFSC0667).
[1] Macrophage
[2] Aging
[3] Autophagy
[4] Kidney Injury