Development of sodium fusidate-loaded microneedle dressing with improved wound healing
Younseop Kim1, Ga-Young Sim1, Chul hyun Park1, Minseok Kang1, Nahyun Kim1, Dongho Lee1, Kyungho Baek Ph.D1,2, Han-Gon Choi Ph.D2, Sung Giu Jin Ph.D1.
1Department of Pharmaceutical Engineering, Dankook University, Cheonan-si, Korea; 2Pharmacy & Institute of Pharmaceutical Science and Technology, Hanyang University, Ansan-si, Korea
Introduction: Microneedle (MN) technology is a promising minimally invasive platform for transdermal drug delivery, but conventional MNs often lack adhesion and fixation. To address this, we developed a sodium fusidate (SF)-loaded double layer MN dressing that improves wound fixation and promotes healing. The SF-loaded tip enables drug delivery, while the base and hydrocolloid backing provide structural support and moisture, as shown in vivo.
Methods:Microneedles were fabricated via vacuum-assisted micromolding using SLA 3D-printed PDMS molds (~1994 μm, 7 × 2 array). The tip layer (500 μL) contained 4% PVA, 4% PVP, 2% chitosan, and 1% lactic acid in 1:1 water:ethanol, followed by vacuum filling and drying. A flexible base (300 μL; 20% PVP, 10% PVA) was added and dried at 40 °C. Hydrocolloid dressings were hot-melt coated (100 °C, 0.5 mm) using SIS, PIB, and PHR. FTIR, DSC, and XRD confirmed amorphization. Drug release and permeation were measured by HPLC. In vivo healing was tested using S. aureus–infected cut or stab wounds in SD rats. Histology used H&E staining.
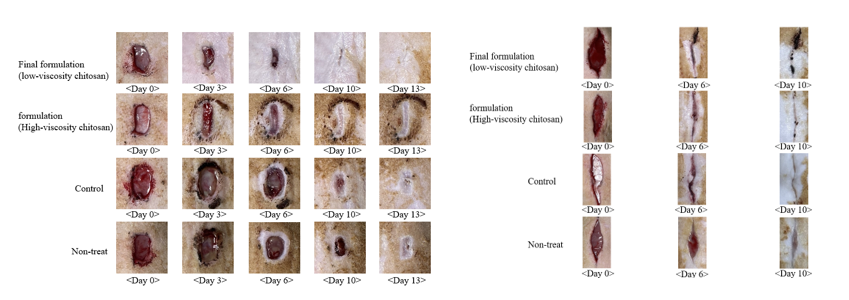
Results: The final formulation (SF:PVP:PVA = 4:10:40; chitosan:lactic acid = 2.5:5) produced sharp, reproducible tips and exhibited the highest mechanical strength among all tested compositions. Low-viscosity chitosan enhanced elasticity and swelling via hydrogen bonding with PVA. This optimized double layer structure showed superior tensile strength, elongation, and conformability. FTIR confirmed SF functional groups; DSC and XRD showed crystalline peak loss, indicating amorphization and improved solubility. The formulation achieved full SF release within 2 hours. Ex vivo porcine skin tests confirmed full insertion and 600 μm penetration. After 24 hours, skin permeation reached 213.45 μg/cm², over 10× commercial fusidic acid gel. In vivo, it achieved wound closure rates of 95.87% (cut) and 99.74% (stab), outperforming commercial patches and gels. Co-application with hydrocolloid dressing further improved healing to 98.6% in a secondary cut model. Histology showed enhanced epithelialization, reduced inflammation, and well-organized granulation tissue, especially in the MN with hydrocolloid group.
Conclusion: This SF-loaded double layer microneedle dressing offers strong mechanical properties, rapid release, enhanced skin permeability, and excellent healing efficacy. It significantly improved wound healing over conventional treatments. With its modular, biocompatible design, it is a promising candidate for next-generation transdermal therapy and shows potential for regenerative medicine and skin tissue regeneration by promoting re-epithelialization and granulation in wound environments.
This work was supported by a National Research Foundation of Korea (NRF) grant under the Korean government (MEST) (No. RS-2023-00208448). .
[1] Micronedle patch
[2] Sodium fusidate
[3] Transdermal drug delivery
[4] Woun healing
[5] Hydrocolloid dressing
[6] IN VIVO wound model