
Graduate Student (MSc)
Department of Surgery
University of Alberta
Transformation of adipose-derived mesenchymal stem cells into parathyroid hormone secreting cells
Illia Levin1, Jeongwoo Kim1, Andrew Poholka1, Brett Wickware1, Daxter Cornand1, Gina Rayat1, David Williams1.
1Surgery, University of Alberta, Edmonton, AB, Canada
Introduction: Normal parathyroid glands, measuring 3–5 millimeters, and their vasculature can be excised or damaged during neck surgeries. Such damage can lead to post-surgical permanent hypoparathyroidism (hypoPT), a condition characterized by insufficient secretion of parathyroid hormone (PTH). PTH is crucial for maintaining calcium homeostasis in the body. Patients with permanent hypoPT suffer from a decreased quality of life and are typically managed with lifelong calcium and calcitriol (active vitamin D) supplementation, which requires constant monitoring and dose adjustment. Incorrect dosing increases the risk of hypercalciuria, kidney stones, and in severe cases, renal failure. Alternative treatments, such as allotransplantation, face challenges of immune-mediated rejection and immunosuppressive drug toxicities, while autotransplantation requires advanced surgical expertise and is limited to well-equipped healthcare systems. TransCon PTH, an FDA-approved recombinant hormone therapy, presents risks of hypercalcemia and a potential risk of osteosarcoma, as observed in animal studies with long-term high-dose administration of this hormone. In this study, we address current treatment limitations for hypoPT by developing a method of transforming adipose-derived mesenchymal stem cells into PTH secreting cells.
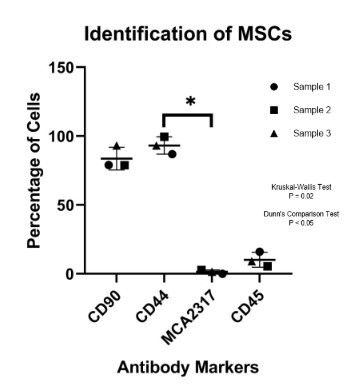
Methods: Porcine adipose-derived mesenchymal stem cells were isolated and characterized by tri-lineage assays and flow cytometry before transforming into PTH secreting cells in media supplemented with 100 ng/mL of Sonic Hedgehog and Activin A for 21 days. PTH gene and protein levels in transformed cells was confirmed using reverse transcription quantitative polymerase chain reaction (RT-qPCR), immunohistochemistry (IHC) and enzyme-linked immunosorbent assay (ELISA), respectively. The gene and protein levels of calcium-sensing receptor (CaSR) was also validated through IHC and RT-qPCR.
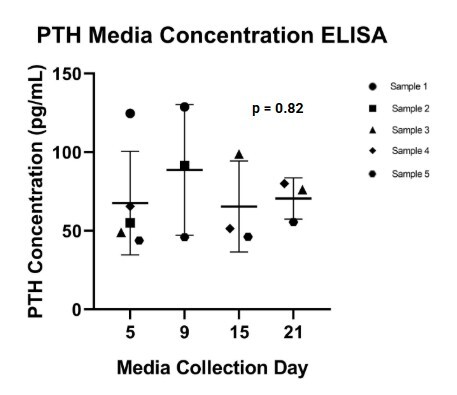
Results: PTH and CaSR gene expression in transformed cells were confirmed by RT-qPCR with ΔCt values of 7.96 and 8.84 respectively. PTH and CaSR proteins presence was validated through IHC staining in transformed cells. ELISA showed the presence of secreted PTH proteins in the culture media by the transformed cells. There were no significant (p = 0.82) differences in the amount of PTH levels detected between 5, 9, 15, and 21 days of transformation.
Conclusion: Our findings demonstrate that porcine adipose-derived mesenchymal stem cells can be successfully differentiated into PTH secreting cells using our method. This study supports the foundation for a cell-based therapy for hypoparathyroidism. Ongoing work focuses on evaluating the dynamic function of PTH secretion in response to different calcium concentrations. Future work includes adopting our method using human adipose-derived mesenchymal stem cells and developing a hypoPT animal model to test the function of the transformed cells in vivo.
University of Alberta Hospital Foundation . Edmonton Civic Employees Charitable Assistance Fund.
[1] Parathyroid Hormone (PTH)
[2] Hypoparathyroidism (hypoPT)
[3] Sonic Hedgehog (SHH)
[4] Adipose-derived mesenchymal stem cells (AD-MSCs)
[5] Enzyme-linked immunosorbent assay (ELISA)
[6] Immunohistochemistry (IHC)
[7] Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
[8] Calcium-sensing receptor (CaSR)
[9] Delta threshold cycle (ΔCt)
[10] Activin A