Anti-CD154 Co-administration Suppresses Antibody Responses and Enhances Safety in Repeated Pig Red Blood Cell Transfusions in Nonhuman Primates
Hee Jung Kang1, Juhye Roh1, Eun Mi Park1, Haneulnari Lee1, Hye Won Lee1, Ju Young Lee2, Jeong Ho Hwang2, Joohyun Shim3, Hyunil Kim3.
1Laboratory Medicine, Hallym University College of Medicine, Anyang-si, Korea; 2Animal Model Research Group, Korea Institute of Toxicology, Jeongeup, Korea; 3Transgenic Animal Research, Optipharm Inc., Cheongju, Korea
Background: Genetically engineered pig red blood cells (pRBCs) have been explored as potential alternatives to human RBCs for transfusion. Transfusions of pRBCs in nonhuman primates (NHPs) provide immediate hematological benefits for up to 24 hours without severe adverse reactions. However, they induce anti-pRBC antibody production, increasing the risk of acute hemolytic transfusion reactions upon subsequent pRBC transfusions. Anti-CD154 antibodies, which block the CD40-CD154 co-stimulation pathway, inhibit B cell activation and subsequent antibody production. This study aimed to determine whether co-administration of anti-CD154 with pRBC transfusions could prevent antibody induction and reduce the risk associated with repeated pRBC transfusions in NHPs.
Methods: Triple knockout (TKO) genetically modified pigs (Optipharm Inc, Korea) provided RBCs for transfusion. Following a withdrawal of 25% of their blood volume, NHPs received TKO pRBC transfusions along with PG-405 (anti-CD154(Fab)-NTIG-IL-10, ProGen, Korea; 30mg/kg, IV), with the same dosage administered again three days later (TKO XTf_aCD154, N = 4). Eight weeks later, after another blood withdrawal, two NHPs received repeated pRBC transfusions with anti-CD154 administration in the same manner (ReXTf_aCD154, N = 2). Blood samples were collected at multiple time points to measure hematological and biochemical parameters, cytokine profiles, complement profiles, and anti-pRBC antibody levels. These results were compared with those from previous experiments without anti-CD154 administration: TKO pRBC transfusions (TKO XTf, N=8) and repeated transfusions (ReXTf, N=2).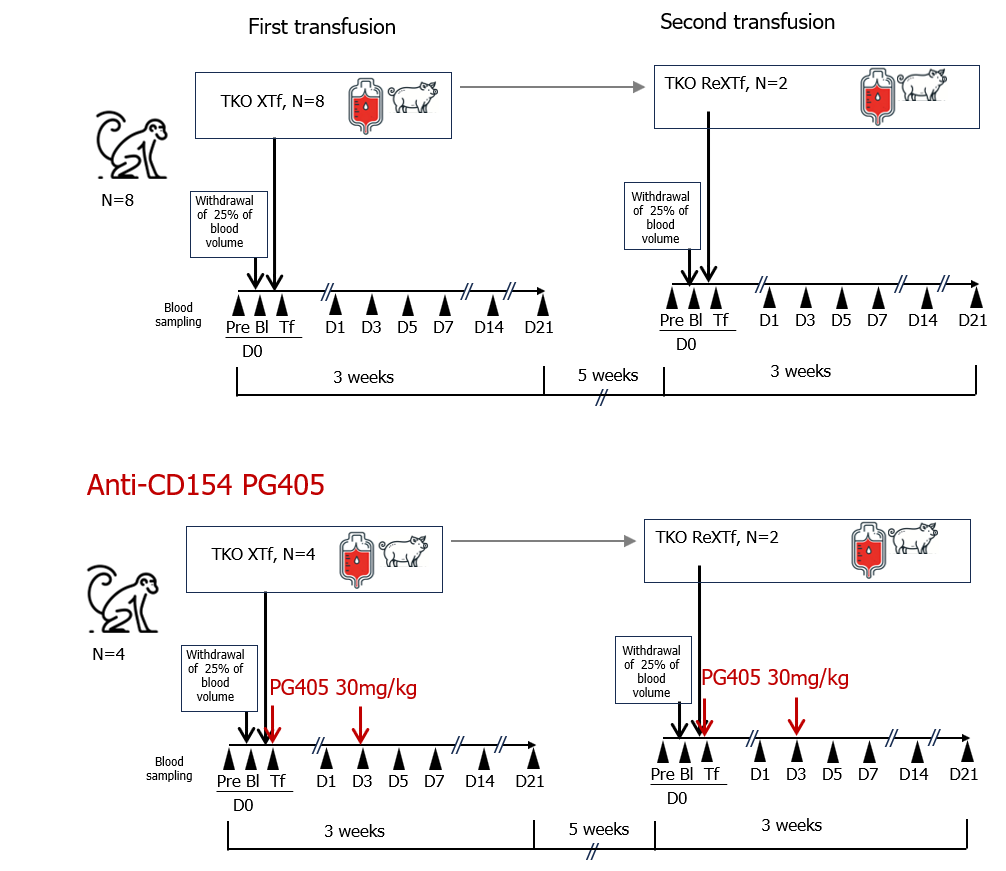
Results: Administration of anti-CD154 did not alter lymphocyte subpopulations in NHPs. No significant differences were observed in hematological, biochemical, or cytokine profiles between TKO XTf and TKO XTf_aCD154 after pRBC transfusions. However, the induction of anti-pig antibodies, which began appearing one week post-transfusion, was significantly suppressed in TKO XTf_aCD154, and their pRBC agglutination titers on post-transfusion day 21 were significantly lower than those in TKO XTf (median titer 2 vs. 128, P=0.0057). During the repeat transfusion, ReXTf_aCD154 recipients showed low pre-transfusion pRBC agglutination titers (median 5.6) compared to ReXTf recipients (median 45). Unlike in ReXTf, immediate hematological benefits lasted for up to 24 hours without any evidence of adverse transfusion reactions in ReXTf_aCD154.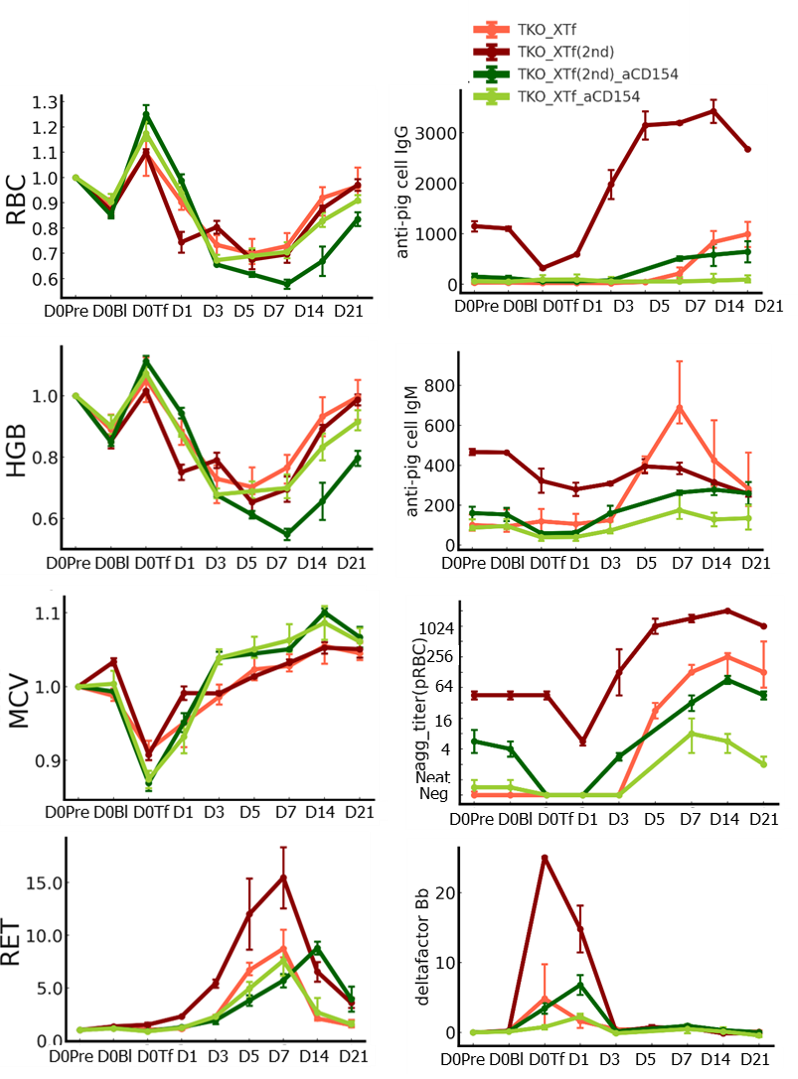
Conclusions: Sensitization and antibody induction following pRBC transfusions are significant hurdles for the clinical application of xenotransfusion. Temporary co-administration of anti-CD154 with pRBC transfusions effectively suppressed anti-pig antibody responses and reduced adverse transfusion reactions upon repeated pRBC transfusions. pRBCs paired with anti-CD154 therapy could be a viable alternative to human RBCs for transfusions. (Grant No. 22-CM-EC-18).
[1] Xenotransfusion
[2] Xenoreactive antibodies
[3] Anti-CD154
[4] Agglutination titers
[5] Nonhuman primates