Research focus: 3D pancreatic differentiation of pluripotent stem cells and ECM-mediated regulation of β-cell development.
Exploiting the properties of human early beta cells to boost generation of insulin producing cells in vitro
Katarzyna Blaszczyk1, Anna P Jedrzejak 1, Natalia Ziojla1, Ekaterina Shcheglova1, Omaima Sabek2, Ashok Balasubramanyam3, Sanjeet Patel4, Malgorzata Borowiak1.
1Institute of Molecular Biology and Biotechnology, Stem Cell Laboratory, Adam Mickiewicz University, Faculty of Biology, Department of Gene Expression , Poznań, Poland; 2Department of Surgery, Methodist Research Institute, Houston, United States; 3Division of Diabetes, Endocrinology and Metabolism, Baylor College of Medicine, Houston, United States; 4Keck School of Medicine, University of Southern California, Los Angeles, United States
Stem Cell Laboratory (Borowiak lab).
Introduction: The endocrine β-cells, are the only cells in the body that can produce insulin, a hormone responsible for controlling glucose metabolism and loss or dysfunction of β-cells leads to diabetes. Currently available therapies require external insulin delivery or glucose-lowering drugs together with lifelong glucose monitoring. Therefore, huge efforts are put into the creation of a robust cell source for β-cells replacement therapy. Data from animal studies and the preliminary findings from human clinical trials indicate that physiological therapy for diabetes could be achieved by transplanting β-cells derived from human pluripotent stem cells (hPSCs). Despite recent progress, hPSCs cannot yet be reliably coaxed into functional β-cells in sufficient purity, number, and costs. The ideal type of cells represents an expandable population of human differentiated β-cells that maintains its functional capacity over long-term.
Methods: hPSCs cells were differentiated towards β-cells using a modified 3D protocol. Cells were treated with recombinant SPOCK2, MMP2, a JNK inhibitor, an MMP2 inhibitor, and mitogens (CHIR99021, Harmine, GW788388, WS6). Proliferation and function were assessed via immunofluorescence, flow cytometry, western blot, immunoprecipitation, zymography, GSIS assays. Transplantation of β-cells was performed under the kidney capsule of SCID-Beige mice. Transcriptomic profiling included single-cell RNA-seq with downstream bioinformatic analysis.
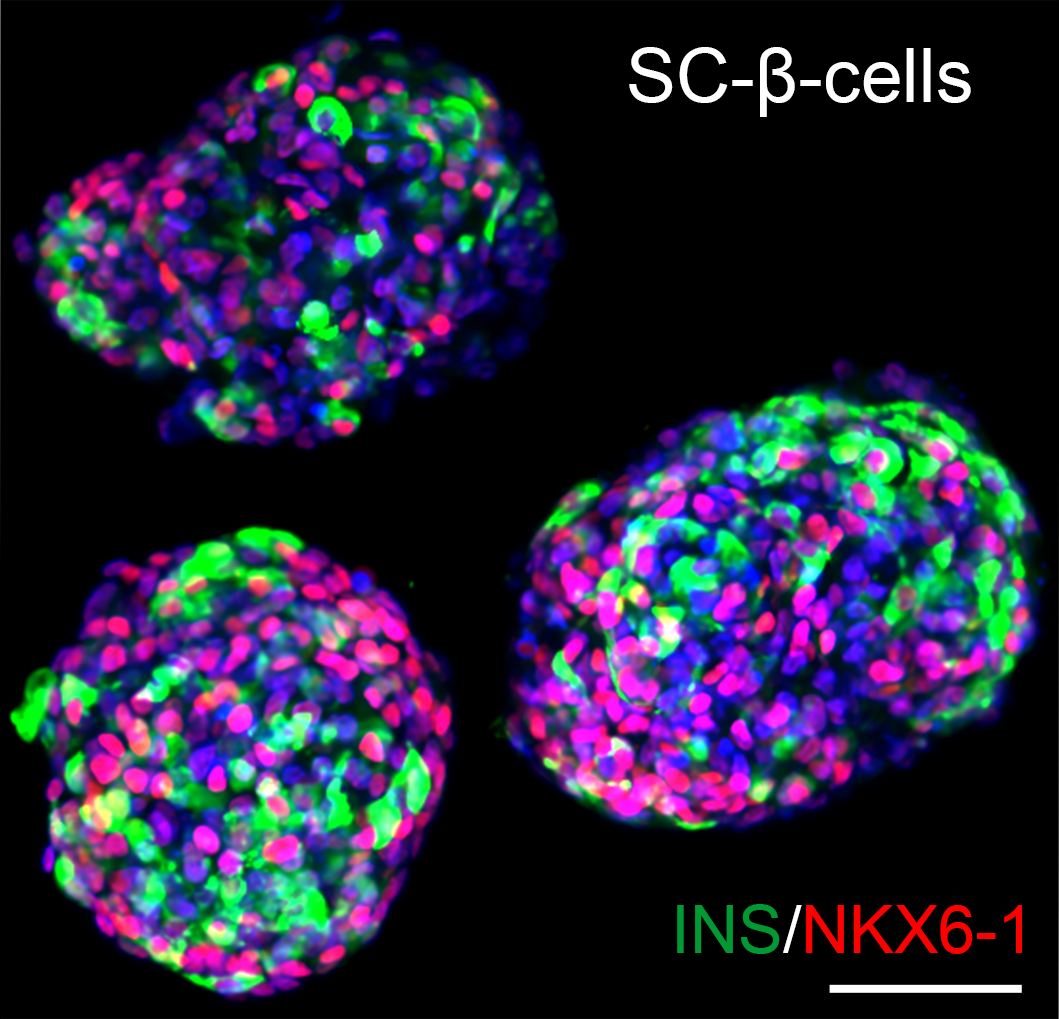
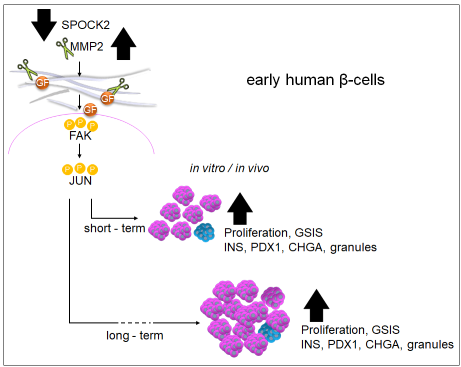
Results: We identified SPOCK2, an extracellular matrix (ECM) protein, as a key inhibitor of immature β-cell proliferation. The proliferating SPOCK2 KO SC-β-cells expressed higher INS, NKX6-1 and PCSK1 levels, suggesting further maturation. Importantly, we showed that SPOCK2 deficiency increases glucose-stimulated insulin secretion (GSIS) from β-cells. SPOCK2 KO SC-β-cells exhibited elevated expression and activity of MMP2, which triggered the β-integrin–FAK–c-JUN signaling pathway. Administration of recombinant MMP2 protein led to substantial short- and long-term expansion of SC-β-cells and markedly improved their glucose-stimulated insulin secretion both in vitro and in vivo with physiological competence comparable to that of human cadaveric islets.
Conclusion: These findings highlight the role of ECM as essential regulator of pancreatic β-cell proliferation, differentiation, and function. Our findings identify SPOCK2, an ECM protein, as a key modulator of fetal β-cell maturation and expansion. In summary, we uncover a molecular mechanism that governs SC-β-cell proliferation and function, pointing to a unique signaling environment that can be leveraged to enhance the generation of fully functional β-cells for therapeutic transplantation.
Foundation for Polish Science. Polish National Science Center . Vivian L. Smith Foundation. Brown Foundation.