Expansion of human intestinal epithelial cells derived from pluripotent stem cells under 2D culture
JunLong Chen1,2, Shinichiro Horiuchi3, So Kuramochi1, Lilika Tabata1, Hidenori Akutsu1, Seiichi Ishida3,4, Akihiro Umezawa1,2.
1Center for Regenerative Medicine, National Center for Child Health and Development, Tokyo, Japan; 2Department of Advanced Pediatric Medicine, Tohoku University School, Miyagi, Japan; 3Division of Pharmacology, National Institute of Health Sciences, Kanagawa, Japan; 4Graduate School of Engineering, Sojo University, Kumamoto, Japan
Introduction: Human small intestinal epithelial cells play a crucial role in drug discovery, including drug screening, pharmacokinetic analysis, and toxicity testing. However, the commonly used Caco-2 cell line, derived from human colon carcinoma, does not adequately recapitulate the physiological functions of the native small intestine. Moreover, primary human small intestinal epithelial cells are limited in availability and subject to donor variability, making consistent supply and quality control difficult. In this study, we generated small intestinal epithelial cells from human pluripotent stem cells using intestinal organoids (mini-gut). We established a two-dimensional (2D) culture system to expand and maintain these cells stably and evaluated their functional stability to assess their potential as a novel model for drug discovery.
Methods: Intestinal epithelial cells were obtained from human pluripotent stem cell-derived mini-guts and co-cultured with mesenchymal stromal cells derived from the same organoids in two-dimensional culture conditions (Figure). Functional characterization was conducted using immunostaining, quantitative real-time PCR (qRT-PCR), RNA sequencing, and electron microscopy. Barrier function (transepithelial electrical resistance and permeability assays), transporter activity (expression and activity of P-gp, BCRP, and PEPT1), and drug metabolic capacity (expression and metabolic activity of CYP3A4) were also assessed.
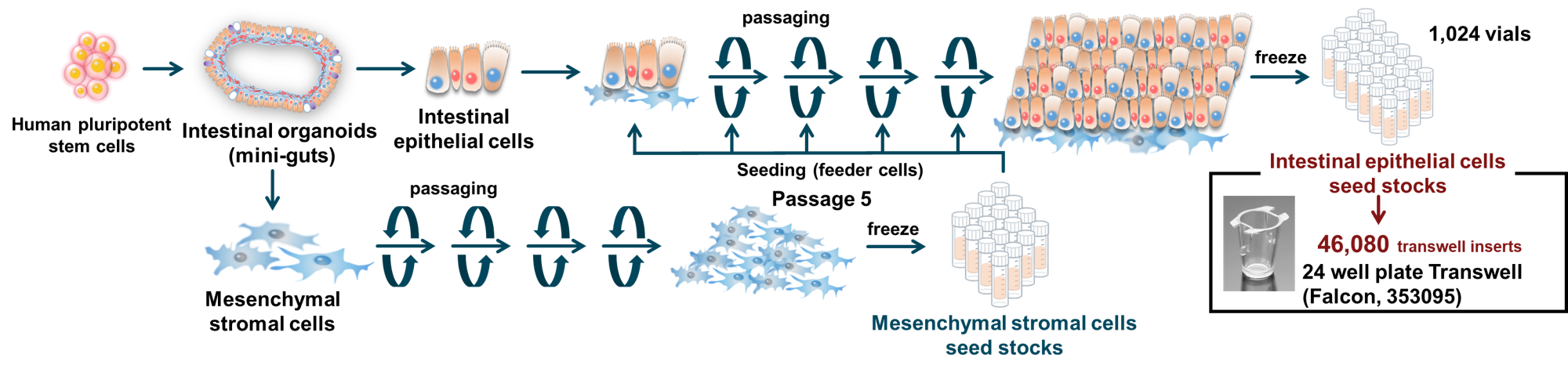
Results: The mini-gut–derived intestinal epithelial cells retained intestinal functions and were capable of serial passage under 2D culture conditions. Co-culture with mini-gut–derived mesenchymal stromal cells was essential for sustaining proliferation and function. The cells exhibited stable proliferation, barrier integrity, transporter activity, and drug metabolic capacity across passages 6 to 9, with no significant differences observed. The mini-gut–derived intestinal epithelial cells’ performance was comparable to or exceeded that of Caco-2 cells. Notably, CYP3A4 expression and activity were similar to those of cryopreserved primary human small intestinal epithelial cells. These cells maintained their functions for over three weeks, during which Ki67-positive proliferating cells remained detectable, suggesting sustained self-renewal. In contrast, at passage 12, cell proliferation declined, and the number of Ki67-positive cells decreased, indicating that the functional limit for usage may be around passage 9.
Conclusion: The mini-gut–derived intestinal epithelial cells exhibit physiologically relevant intestinal functions and can be expanded and cryopreserved in a two-dimensional culture system. These cells demonstrate high reproducibility and stability, offering advantages in ease of handling comparable to those of Caco-2 cells. Given their superior functional fidelity, they hold promise as a next-generation in vitro model for the human small intestine in drug discovery research.
Kayoko Saito. Minoru Ichinose.
[1] Intestinal epithelial cells
[2] Mesenchymal stromal cells
[3] 2D culture
[4] Co-culture
[5] Pluripotent stem cells