
Long renal tubule-like structures can be spontaneously generated from spheroids molded by urine-derived human adult renal progenitor cells.
Angela Picerno1, Francesca Giannuzzi1, Francesca Montenegro1, Antonella Cicirelli1, Alessandra Stasi1, Rossana Franzin1, Loreto Gesualdo1, Fabio Sallustio1.
1Department of Precision and Regenerative Medicine and Ionian Area, University of Bari , Bari, Italy
Introduction: Renal spheroids and organoids derived from stem cells represent effective models for drug testing, nephrotoxicity assessment, and studies on renal development and pathology. However, these systems typically require complex differentiation protocols and sequential growth factor administration. Here, we demonstrate for the first time that adult human renal progenitor cells (ARPCs), isolated from urine of healthy subjects or IgA Nephropathy (IgAN) patients, can spontaneously form spheroids and tubular-like structures, reproducing IgA1 deposits.
Methods: ARPC-derived spheroids and tubular-like structures were generated using a mixed ARPC population and characterized via immunofluorescence, flow cytometry, and ELISA with markers including CD133, NanoG, Oct3/4, GATA-3, SSEA4, CD249, Aminopeptidase N, ZO-1, Uromodulin, and Lotus. Renin secretion was assessed by ELISA; angiogenic potential by CAM assay. PKH-26 labeling was used for tracking. Spheroids cultured with serum from healthy and IgAN patients were tested for IgA1 deposits using immunofluorescence.
Results: ARPC spheroids generated long tubular-like structures without external chemokines, expressing markers of renal tubules and resembling nephron segments (proximal/distal tubules, loop of Henle). These structures secreted renin and exhibited angiogenic activity. When exposed to serum from IgAN patients, ARPC spheroids developed IgA1 deposits at 4, 8, and 15 days (p=0.004, p=0.0029, p=0.0012, respectively). No deposits formed with inactivated IgAN serum or spheroids from healthy donors.
Conclusions: Urinary ARPCs can self-assemble into renal spheroids and differentiate into tubular-like structures without exogenous chemokines. 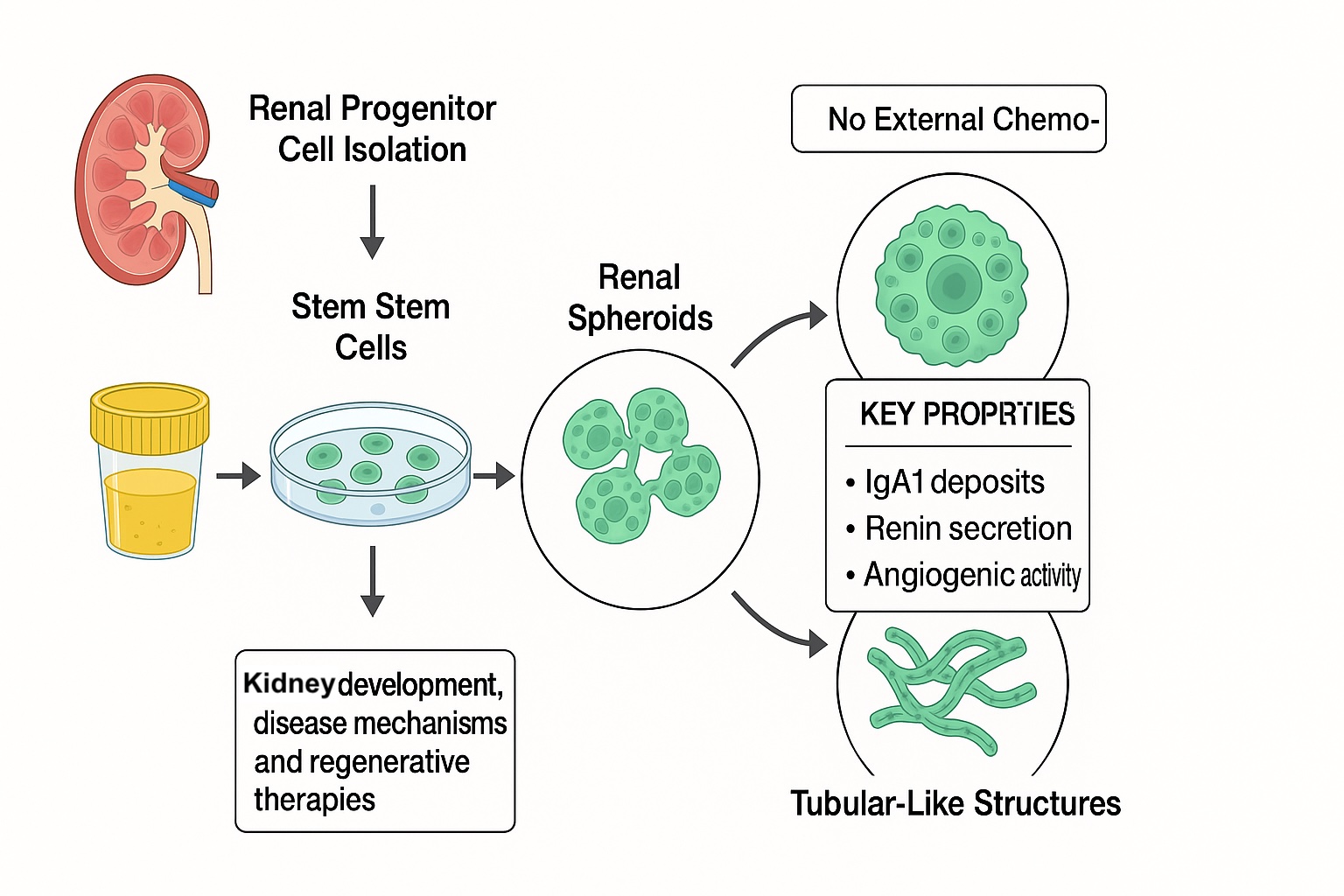 This discovery offers a promising and accessible model for investigating kidney development, disease mechanisms, and innovative regenerative therapies for renal disorders.
This discovery offers a promising and accessible model for investigating kidney development, disease mechanisms, and innovative regenerative therapies for renal disorders.