
Abhiraj Kesharwani is a Postdoctoral Researcher at the University of Tokyo, where he focuses on developing vascularized bone organoids using human pluripotent stem cells and organ-on-a-chip systems to model bone development and diseases. He completed his Ph.D. in Bioengineering at the University of Tokyo in March 2025, supported by the JICA FRIENDSHIP Scholarship. His doctoral research involved studying bone-vascular interactions and tissue development using a microfluidic chip to investigate vascularized bone organoids. With a background in bioengineering, pharmacy, and bioinformatics, Abhiraj aims to bridge stem cell biology and microengineering to advance human disease modeling and translational research.
Modeling early vascular-mesenchymal interactions in human endochondral ossification using human embryonic-stem-cell-derived organoid on a microfluidic chip
Abhiraj Kesharwani1, Shoichiro Tani1,2, Masaki Nishikawa3, Yasuyuki Sakai3, Hiroyuki Okada1,2, Shinsuke Ohba5, Ung-il Chung1,4, Hironori Hojo1,4.
1Center for Disease Biology and Integrative Medicine, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan; 2Department of Orthopaedic Surgery, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan; 3Department of Chemical Systems Engineering, Graduate School of Engineering, The University of Tokyo, Tokyo, Japan; 4Department of Bioengineering, Graduate School of Engineering, The University of Tokyo, Tokyo , Japan; 5Department of Tissue and Developmental Biology, Graduate School of Dentistry, Osaka University, Osaka, Japan
Introduction: Vascular interactions are essential for developing tissues during embryogenesis, particularly in skeletal development through endochondral ossification. During this process, mesenchymal cells undergo condensation to give rise to skeletal elements, while simultaneously interacting with newly formed vascular networks. These skeletal elements are later invaded by the vasculature, initiating bone marrow formation. While we and other groups established an in vivo model for endochondral ossification using human embryonic stem cell (hESC)-derived sclerotome (osteochondral progenitors) implanted in immunodeficient mice have successfully recapitulated key aspects of it (Cell Rep. 2023). However, given the physiological differences between mouse and human systems, there remains a need for in vitro platforms that can more precisely model early-stage, human-specific vascular-mesenchymal interactions during endochondral ossification.
Methods: To bridge this gap, we utilized a commercial organ-on-a-chip platform based on a 64-chip microfluidic device (OrganoPlate® Graft) to replicate vascular-mesenchymal interactions relevant to early endochondral ossification (Fig.1). Each chip consists of 1 in-gel culture channel, 2 perfusion channels, and an open graft chamber. These perfusion channels mimic physiological flow conditions critical for vascular sprout formation. Out of the tested extracellular matrices, fibrin gel supported significantly more robust vascular-like network formation by mCherry-labeled human umbilical vein endothelial cells (HUVECs) compared to conventional collagen-I gel. The perfusability of these networks was demonstrated using fluorescein isothiocyanate (FITC)-dextran and fluorescent microbeads, indicating the establishment of functional vascular-like networks.
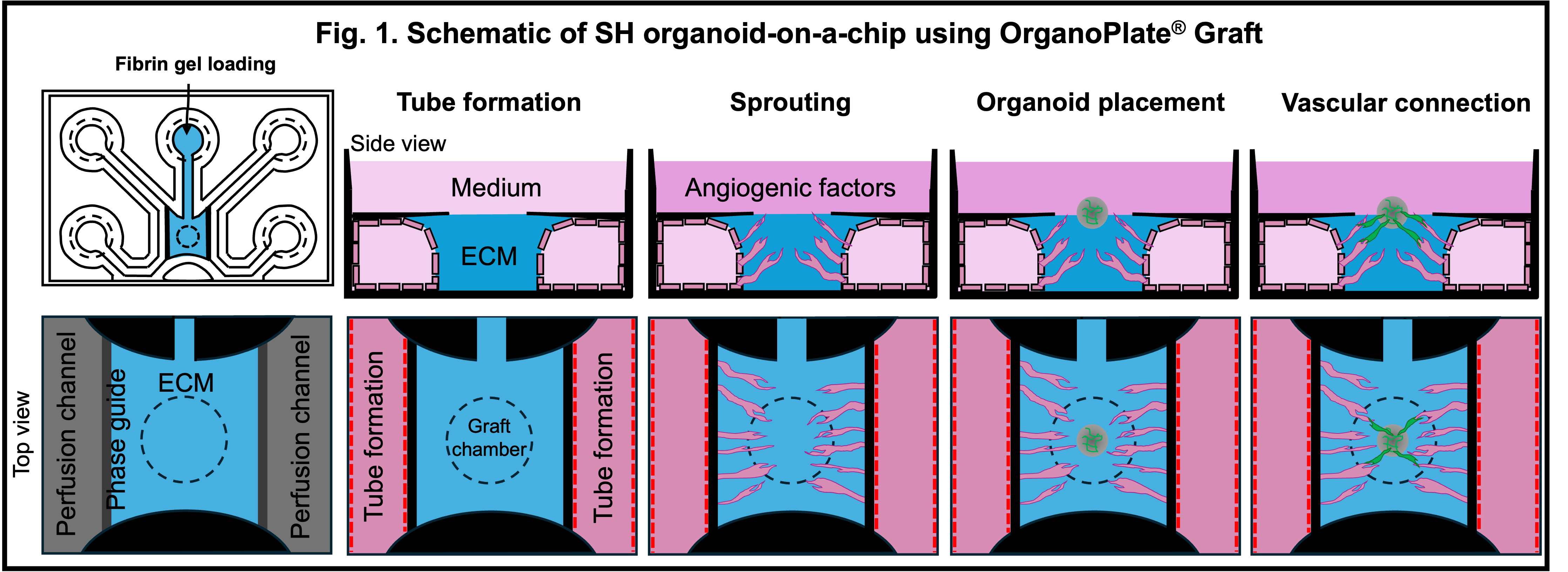
Results: To model mesenchymal condensation during the initial stage of endochondral ossification and its interaction with vascular networks, we mixed hESC-derived sclerotome and enhanced green fluorescent protein (EGFP)-labeled HUVECs and introduced this mixture into the chip, which we termed SH organoid. These SH organoids sustained vascular-like networks formed by mCherry-HUVECs with greater longevity than sclerotome spheroids alone. Notably, EGFP-HUVECs migrated outward from the organoids and contributed to the formation of secondary vascular-like structures, which partially connected with the pre-existing mCherry-labeled vascular-like networks (Fig.2). After 7 days in culture, SH organoids exhibited SOX9 expression in the condensed mesenchymal core and type I collagen in a perichondrial-like outer layer, recapitulating early events of physiological endochondral ossification.
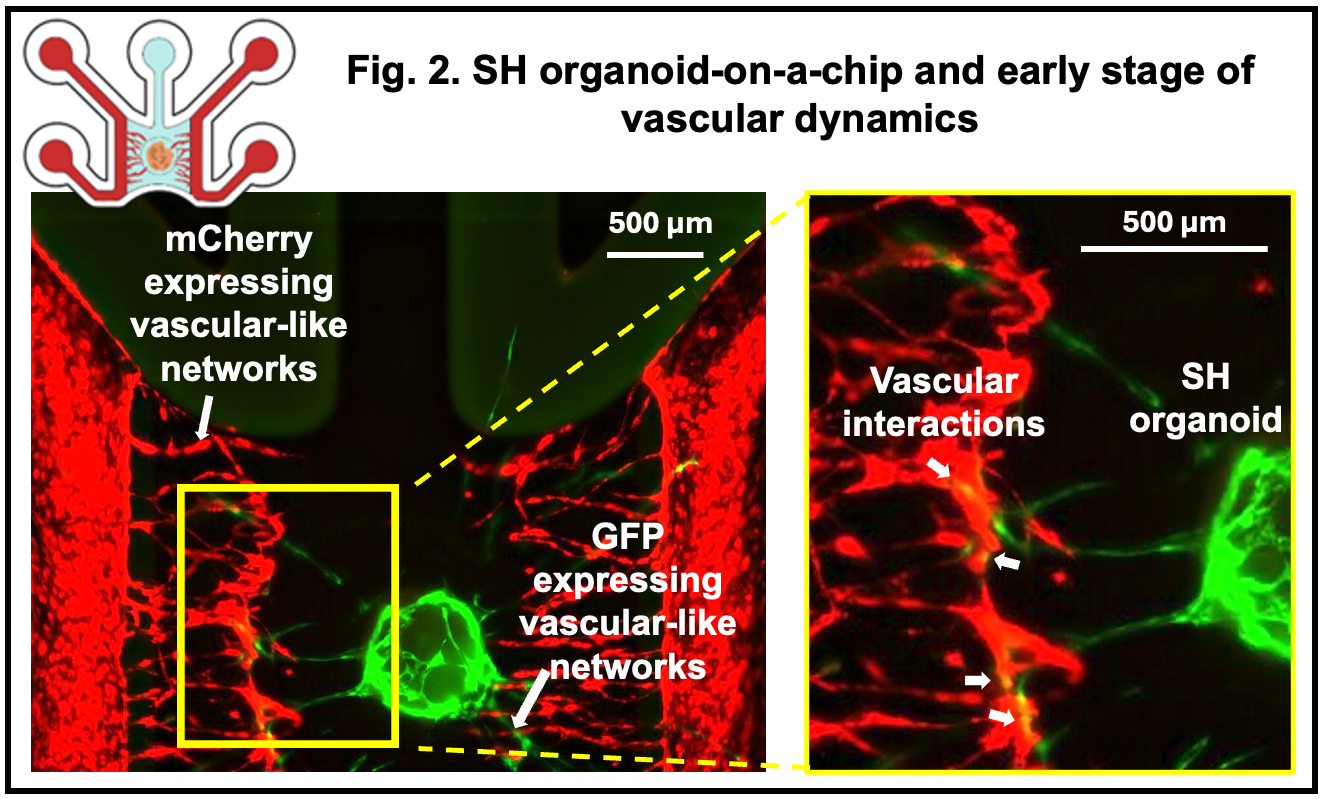
Conclusion: This SH organoid-on-a-chip model represents an in vitro system that mimics the early vascular microenvironment of human skeletal development. Beyond developmental studies, this platform holds potential for investigating the impact of vascular dynamics on early ossification and for modeling genetic skeletal disorders such as campomelic dysplasia (SOX9 mutations) and thus may contribute to the discovery of targeted therapeutic strategies.
Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS: 21H04952). Rising Star Award from American Society for Bone and Mineral Research. The Japan Science and Technology Agency (JST) FOREST Program (JPMJFR225N). JST ERATO program (JPMJER2401). Research Grant from Takeda Science Foundation.
[1] Endochondral ossification
[2] Vascular interaction
[3] Human embryonic stem cells
[4] Sclerotome cells
[5] Microfluidic platform
[6] Organoid-on-a-chip