
Dr. Ren is an Associate Professor of Biomedical Engineering at Carnegie Mellon University, and Director of the Engineering Morphogenesis Group. Dr. Ren received his B.S. in Biological Sciences and Ph.D. in Cell Biology, both from Peking University. He completed his post-doctoral training in tissue engineering at Harvard Medical School. Current research in his group focuses on investigating fundamental cell-cell and cell-ECM (extracellular matrix) interactions during the biofabrication of lung and vascular tissues, and are funded by grants from the NSF, NIH, DoD, ARPA-H, and private foundations. Dr. Ren received the NSF CAREER Award, Rising Star Award from the Biomedical Engineering Society, Biomedical Engineering New Innovator Award from the Northeast Bioengineering Conference, and was named Dean’s Early Career Fellow by the College of Engineering at Carnegie Mellon University. He serves on the Young Investigator Committee at the Cell Transplant and Regenerative Medicine Society, and on the Publication Committee at the American Society of Matrix Biology.
Engineering motile airway organoid and assembloid for therapeutic development and delivery
Xi Ren1.
1Department of Biomedical Engineering, Carnegie Mellon University, Pittsburgh, PA, United States
Introduction: Due to exposure to airborne pollutants, respiratory diseases are on the rise worldwide, including chronic obstructive pulmonary disease, bronchiectasis and asthma. These diverse disorders have a pathological hallmark in common: cilia beating defects (ciliopathy), leading to impaired mucociliary clearance and airway obstruction. Thus, there is a critical need of technologies to enable effective and accelerated assessment of respiratory ciliopathy from patient-derived samples to expediate therapeutic development and personalized disease management.
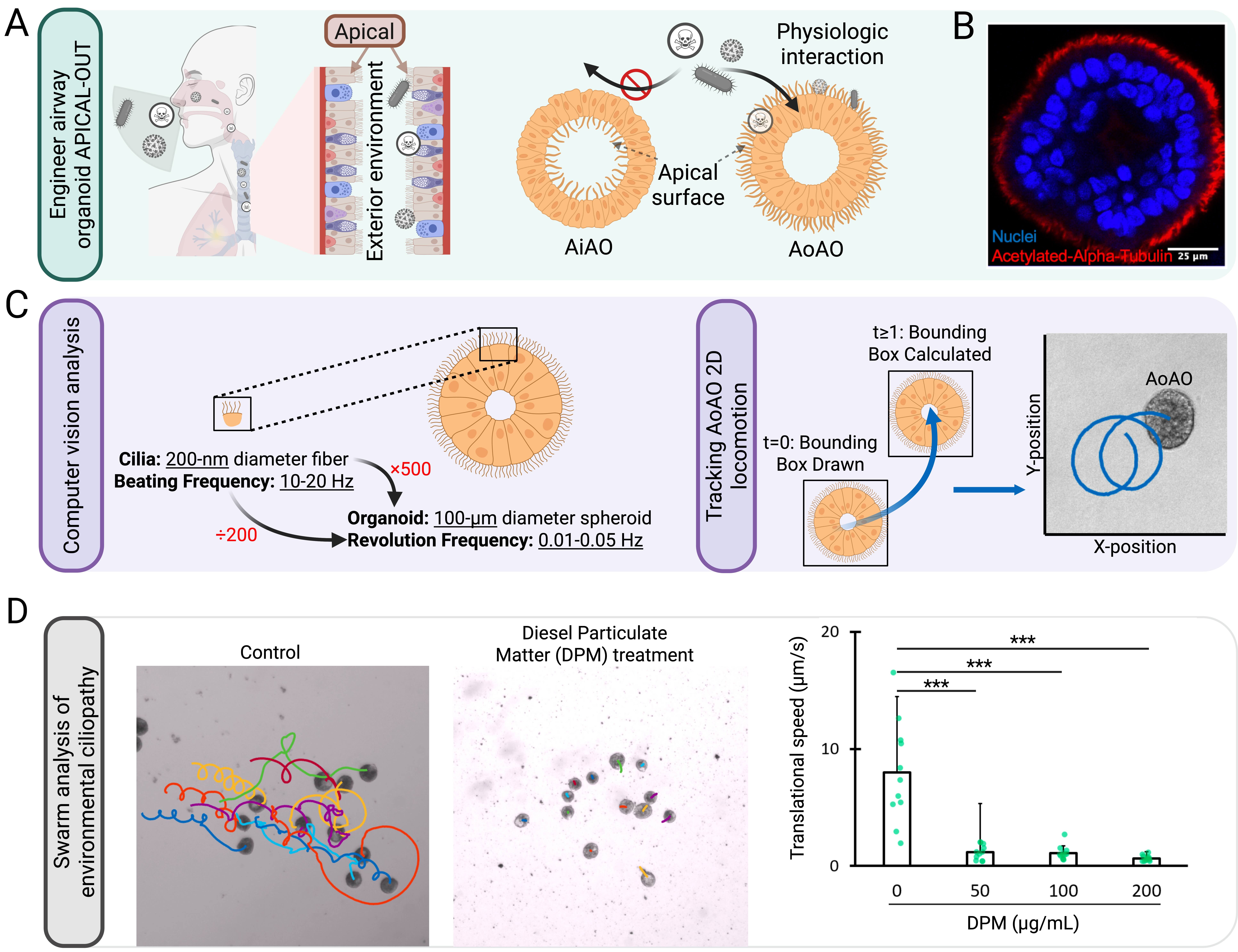
Methods: A novel extracellular matrix-free suspension culture was developed for human airway organoid engineering to effectively reverse the epithelial polarity to an apical-out conformation, delivering Apical-out Airway Organoid (AoAO, Fig 1A), which became motile propelled by the exterior-facing cilia beating. Accordingly, we developed computer vision algorithms to reliably track AoAO motility and performed correlation studies with direct kymograph analysis of cilia beat frequency. We then assessed the feasibility of the AoAO platform for modeling genetic and environmentally induced ciliopathy. Lastly, we leveraged spatially controlled tissue aggregation to build larger-scale apical-out airway assembloid systems to diversify tissue geometry and functional cilia distribution, and characterized how these new morphological levers modulate the overall tissue locomotion.
Results: Over three weeks of differentiation, AoAO underwent effective mucociliary differentiation presenting homogenous motile cilia on its exterior surface (Fig 1B). Coordinated cilia beating powers AoAO locomotion in 2D and 3D, offering a unique opportunity to study ciliary health with a net, tissue-level functional output. Our computational algorithms transformed high-frequency, nanoscale ciliary beating into low-frequency, microscale organoid locomotion, augmenting both spatial and temporal resolution (Fig 1C). Using EHNA, an inhibitor of molecular motor protein dynein that drives cilia beating, we established the quantitative correlation between the individual cilia beat frequency and translational velocity of AoAO locomotion. We further showed that the AoAO model was responsive to pro-inflammatory stimulations induced by either cytokine (IL-13) or model pollutant (diesel particulate matter) (Fig 1D). Lastly, we demonstrated a guided modular tissue aggregation pipeline for fabricating AoAO with diversified tissue geometries (rod/triangle/diamond-shaped) and resulting locomotion patterns.
Conclusion: Here we established the AoAO as a robust, high-throughput tissue platform for modeling airway pathophysiology and screening potential therapeutics. We also envision AoAO’s tunable geometry and locomotion will lay the groundwork for engineering patient-derived living robotic pharmacy to be deployed in vivo to express and deliver desired therapeutic cargos (such as recombinant proteins/enzymes) to combat respiratory diseases.
This work is supported by Unites States Department of Defense Peer-Reviewed Medical Research Program W81XWH2110183, and Manufacturing Futures Institute at Carnegie Mellon University. .
[1] Organoid
[2] Assembloid
[3] Motile Cilia
[4] Computer Vision
[5] Living Pharmacy