
Pathologist at Policlinico di Milano, specializing in renal, cutaneous, pulmonary and transplant pathology including surgical specimen analysis, autopsy, histological, cytological, and molecular pathology diagnostics.
Active in research and medical writing, with contributions to non-neoplastic pathology and translational oncology. Experienced in leading multidisciplinary research teams, driving innovation in pathology.
Tubular miRNA stress signature defines the principal ischemic axis in DCD kidneys: A compartment-resolved molecular study with implications for graft assessment
Nadia Mansour1, Giorgia Bianchi1, Alessandra Maria Storaci1, Marco Brevi1,5, Massimo Cardillo2, Maria Carmela Rossi2, Stefano Ferrero1,5, Giuseppe Castellano3, Valentina Vaira1,4, Alessandro Del Gobbo1.
1Division of Pathology, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milano, Italy; 2SC Trapianti Lombardia, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milano, Italy; 3SC Nefrologia e Dialisi, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milano, Italy; 4Dipartimento di Scienze Biomediche, Chirurgiche e Odontoiatriche, Università degli Studi di Milano, Milano, Italy; 5Dipartimento di Fisiopatologia Medico-Chirurgica e dei Trapianti, Università degli Studi di Milano, Milano, Italy
Background: Kidneys from donation after circulatory death (DCD) are more susceptible to ischemia-reperfusion injury. Given their regulatory role in hypoxia, fibrosis, and cell death, miRNAs may offer compartment-specific markers to predict graft viability. We hypothesized that renal tubules from DCD donors represent the critical target of ischemic damage and aimed to test this through high-resolution miRNA profiling.
Methods: We retrospectively analyzed renal tissue collected from donation after brain death (DBD) (n =19) and DCD (n = 18) donors. Glomerular and tubular compartments were isolated by laser-capture microdissection for compartment-specific microRNA profiling. A panel of hypoxia- and EMT-related miRNAs was analyzed by qPCR. Differential expression was assessed with Benjamini-Krieger-Yekutieli FDR correction (q value <0.05). Functional interpretation of miRNAs was integrated based on experimentally validated targets and pathway annotations.
Results: In DBDs, glomeruli showed upregulation of miR-145 (FC= 1.90, q = 0.000003), miR-210 (FC=0.82, q = 0.003), and miR-424 (FC=1.45, q = 0.019), indicating a hypoxia-driven but vascularly competent state. Tubules from the same DBD samples exhibited marked downregulation of miR-107 (FC= –3.58, q = 0.003), consistent with a robust metabolic stress response. These differences suggest a common mechanism of adaptation to stress and a compartment-specific deregulation of miR-107.
In DCDs, a more pronounced divergence between compartments was observed. Glomeruli in DCD were characterized by upregulation of miR-145 (FC=2.30, q=0.000004) and miR-210 (FC=3.03, q = 0.017), alongside significant downregulation of miR-429 (FC=–2.94, q < 0.000001) and miR-107 (FC=–4.08, q = 0.00001), suggesting an EMT-promoting signaling. In contrast, DCD tubules showed a marginal increase of miR-107 (FC=1.14, p = 0.016, q = 0.1). Notably, this is the only DCD compartment with miR-107 upregulation, underscoring its role as a central mediator of ischemic injury (Figure 1).
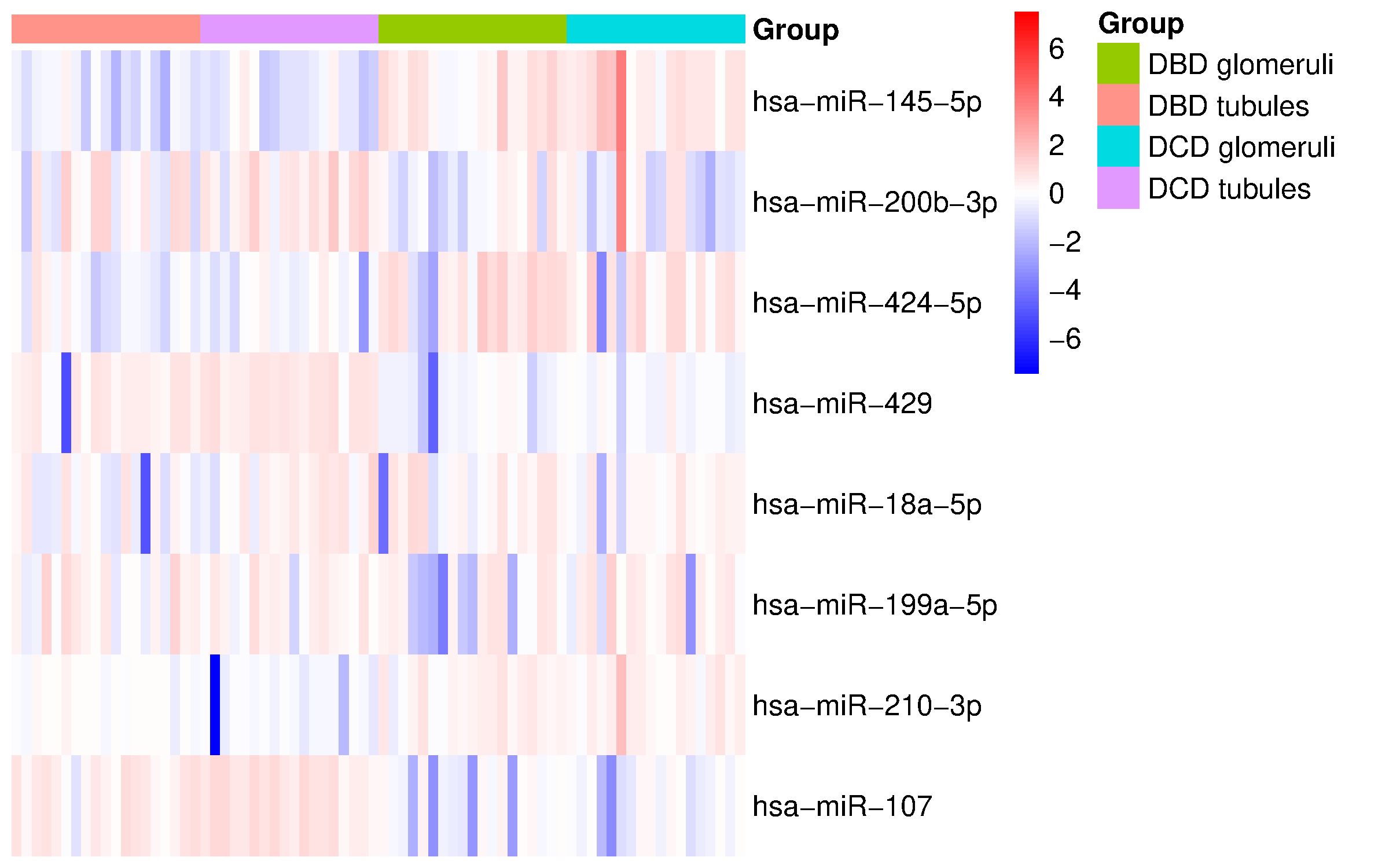
Moreover, direct comparison of DCD and DBD glomeruli revealed no miRNAs reaching statistical significance, reinforcing the concept of relative glomerular resilience and highlighting tubules as the primary site of injury in DCD grafts.
Conclusions: Our results define the DCD tubular compartment as the dominant source of ischemic molecular stress, with miR-107 emerging as a robust sensor of metabolic and hypoxic damage. These findings suggest that histological and molecular assessment of DCD grafts should focus on the tubular compartment to more accurately stratify graft quality. Integrating miRNA-guided diagnostics into pre-transplant evaluation protocols may improve donor organ assessment, particularly in marginal donor programs. While our findings provide a molecular characterization of graft status, further studies are needed to correlate specific miRNA signatures with early injury markers and long-term graft function.
[1] Donor after cardiac death
[2] Graft quality
[3] MicroRNA profiling
[4] Pre-transplant assessment
[5] Compartment-specific biomarkers
[6] Ischemia-reperfusion injury