Ammonia detoxification inhibits Tregs apoptosis by Urea cycle to suppress autoimmune diseases
Yu Li1, Jian Gu1, Ling Lu2.
1Department of Hepatobiliary Surgery, The First Affiliated Hospital of Nanjing Medical University, Nanjing, People's Republic of China; 2Department of Hepatobiliary Surgery, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, People's Republic of China
Ling Lab.
Introduction: Liver transplantation is currently the most effective therapeutic approach for end-stage liver diseases and liver failure; however, transplant rejection significantly impacts its prognosis. Although conventional immunosuppressive agents are widely used clinically, long-term administration often leads to severe side effects. Hence, there is an urgent need to explore novel, safe, and effective immunomodulatory strategies to induce immune tolerance in transplantation. Regulatory T cells (Tregs), as critical immunosuppressive cell subsets, play a central role in maintaining immune homeostasis and inducing transplant immune tolerance. Therefore, effectively regulating the function of Tregs can suppress transplant rejection. Metabolic pathways significantly modulate the function and stability of Tregs, especially through the accumulation of metabolites in the local microenvironment, profoundly influencing Treg immunosuppressive activity. Recent studies indicate that ammonia, previously considered mainly cytotoxic, may play a positive role in regulating immune cell function. Our preliminary studies found that appropriate concentrations of ammonia can significantly enhance Treg function and immunosuppressive activity, effectively reducing transplant rejection. Based on these findings, this study will explore the specific mechanisms of ammonia metabolism regulating Tregs and further evaluate the therapeutic potential of ammonia metabolism-regulated Tregs in GVHD models.
Methods: Mass cytometry was utilized to assess the impact of ammonia diets on the immune microenvironment in mice. In vitro experiments were conducted to evaluate the effects of ammonia on various immune cells. Additionally, ammonia diets were tested therapeutically in different autoimmune disease models to assess their regulatory effects on immune tolerance.
Results: Mass cytometry revealed that ammonia diets elevated serum ammonia levels and significantly increased Treg proportions in mice. Additionally, ammonia diets enhanced Treg immunosuppressive functions 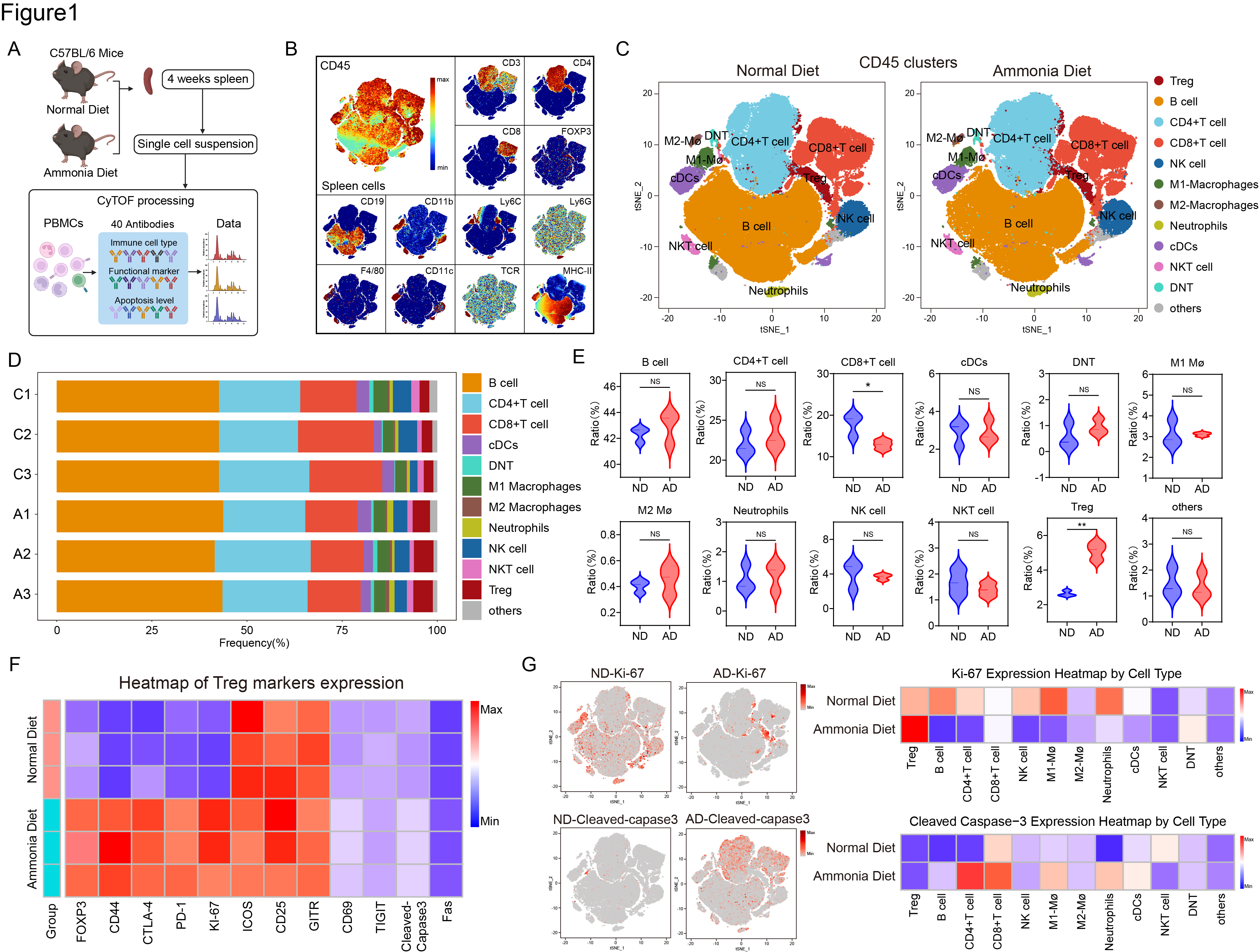 .In vitro experiments showed that ammonia treatment induced apoptosis in various T-cell subsets, while Tregs exhibited resistance to ammonia cytotoxicity at certain concentrations due to their high expression of argininosuccinate lyase (ASL) and robust ornithine cycle activity. Furthermore, ammonia was found to bind to SRC3, highly expressed in Tregs, enhancing its interaction with STAT3, thus promoting STAT3 phosphorylation and ASL transcription.
.In vitro experiments showed that ammonia treatment induced apoptosis in various T-cell subsets, while Tregs exhibited resistance to ammonia cytotoxicity at certain concentrations due to their high expression of argininosuccinate lyase (ASL) and robust ornithine cycle activity. Furthermore, ammonia was found to bind to SRC3, highly expressed in Tregs, enhancing its interaction with STAT3, thus promoting STAT3 phosphorylation and ASL transcription.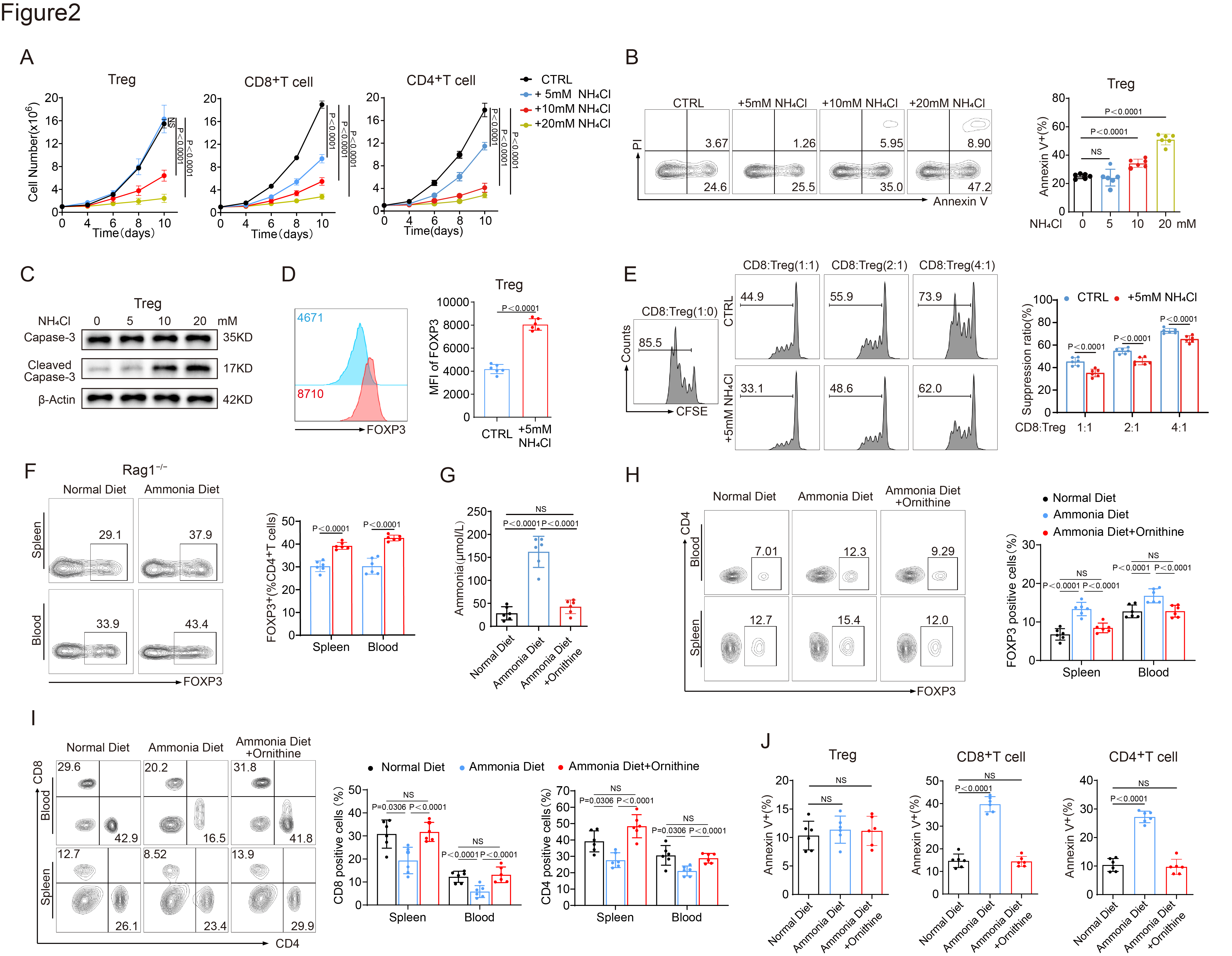 .
.
Conclusion: Ammonia induces apoptosis of immune cells in transplanted livers; however, Tregs sense ammonia through the SRC3-STAT3 complex, initiating ornithine cycle metabolism to detoxify ammonia and enhance their immunosuppressive function. Ultimately, metabolic remodeling of the immune microenvironment through the suppression of effector T cells and enhancement of Tregs facilitates clinical immune tolerance post-liver transplantation.
This study was supported by grants from the National Natural Science Foundation of China (81971495, 82171759, 82101873), the CAMS Innovation Fund for Medical Sciences (2019-I2M-5-035)..
[1] Immunosuppression
[2] Regulatory T cells
[3] Autoimmune Disease
[4] Urea cycle
[5] Ammonia Detoxification