Carola Davila is a Research Professional II at the University of Arizona in the Department of Surgery Insititute for Cellular Transplantion. She has been at the U of A for four years and is currently participating as an human islet isolation lead for her program. Carola is also a Cellular and Molecular Medicine master's student at the U of A.
hSC-islets maintain their viability and function when shipped using gas-permeable silicone rubber membrane vessels
Carola Davila1, Amy C Kelly1, Farida Sarangi2, Jennifer P Kitzmann1, Craig S Weber1, Cristina Nostro2, Thomas Loudovaris 1, Charles W Putnam 1, Klearchos K Papas1.
1Surgery, University of Arizona, Tucson, AZ, United States; 2McEwen Stem Cell Institute , University Health Network, Toronto, ON, Canada
Introduction: Human stem cell-derived islets (hSC-islets), offer a potentially unlimited supply of insulin-producing cells for large scale implementation of ß-cell replacement therapy. A key logistical consideration for deploying hSC-islets in a large number of geographically distant clinical or research settings is the ability to ship these cells from specialized manufacturing facilities while maintaining their viability and function. Convenient and frequently used shipment methods, such as oxygen-impermeable conical tubes, have shown to significantly compromise cell quality especially when shipped at ambient temperature. In this study, we demonstrate that hSC-islets can be shipped at room temperature using gas-permeable silicone rubber membrane vessels with high functionality and viability measured upon receipt.
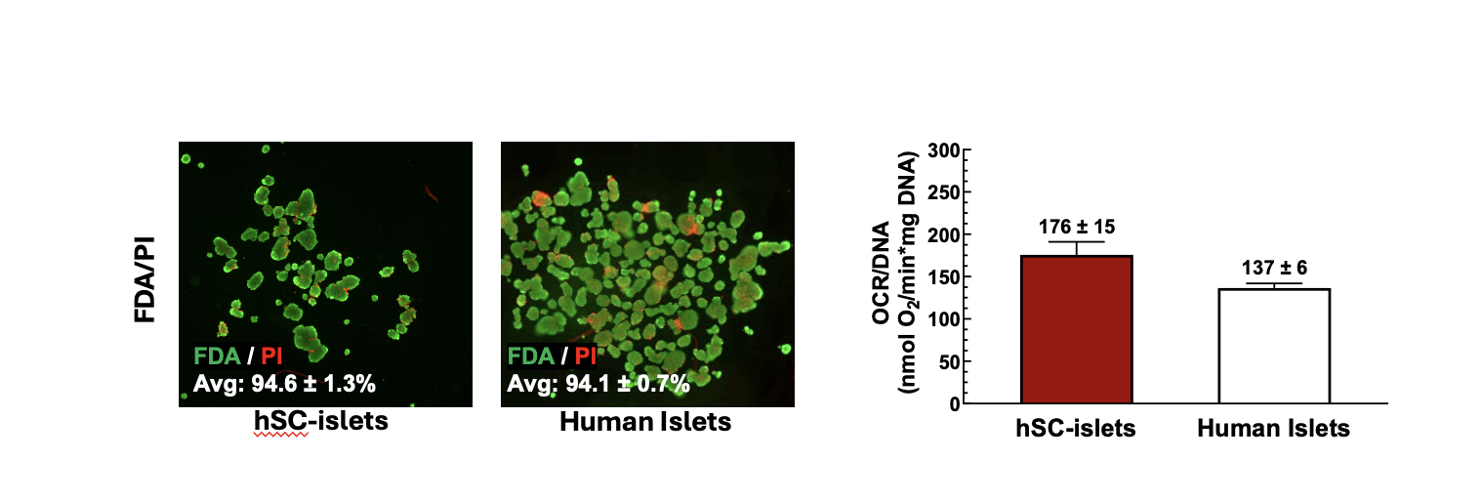
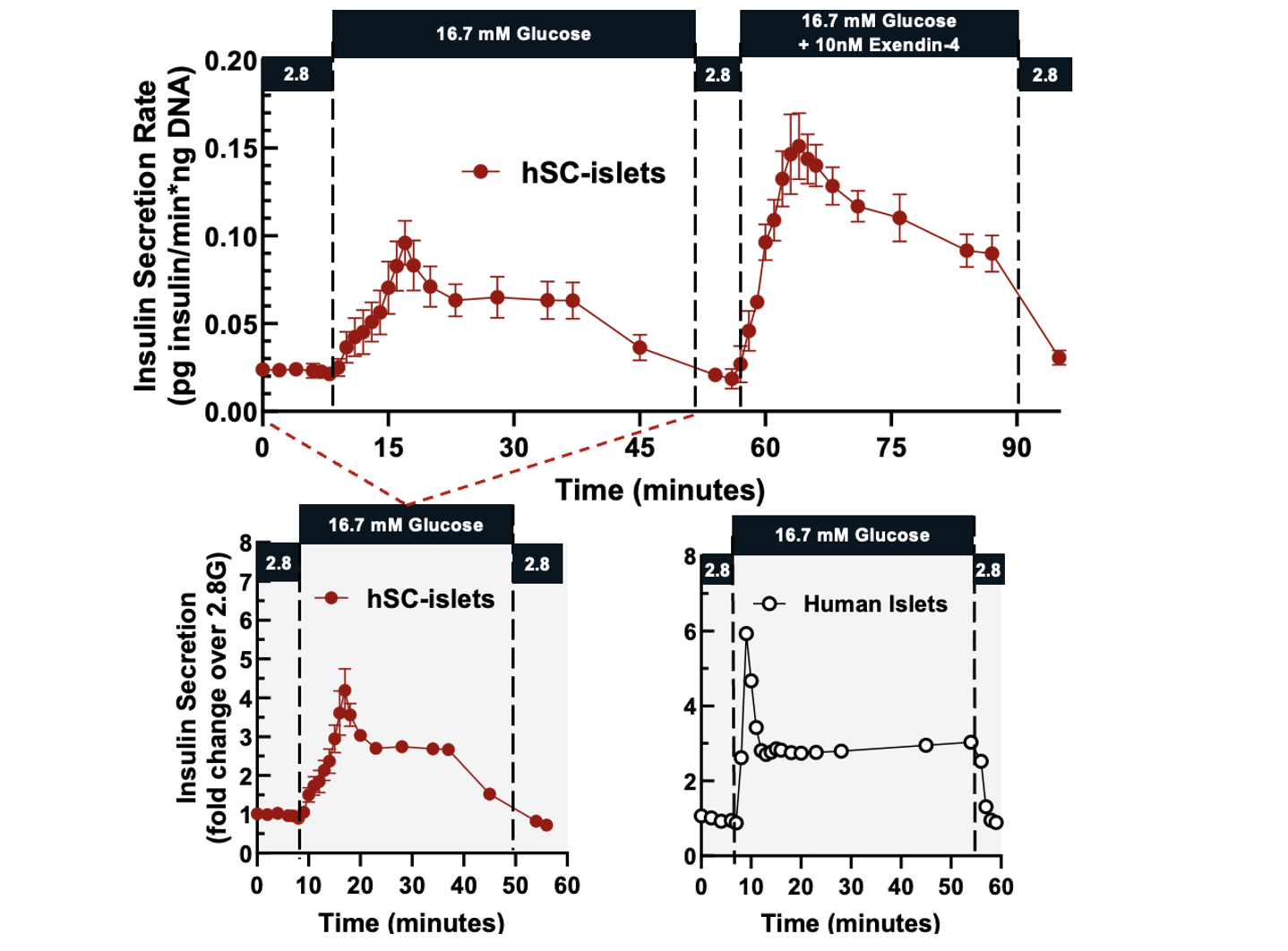
Methods: hSC-islet aggregates were differentiated in Dr. Nostro’s lab (Toronto, Canada) and shipped to Tucson, Arizona, USA (n=5 independent shipments) using silicone rubber membrane vessels (G-Rex®, Wilson Wolf). Vessels were placed in boxes within a Styrofoam cooler along with phase change materials. Upon receipt, hSC-islets were assessed for function by dynamic perifusion (Biorep Perifusion System), viability by membrane integrity staining (fluorescein diacetate / propidium iodide: FDA/PI), and metabolic activity (oxygen consumption rate normalized to DNA content: OCR/DNA).
Results: hSC-islets shipped internationally using silicone rubber membrane vessels arrived as intact aggregates and were viable, metabolically active, and highly functional. Membrane integrity assay showed high viability (94.6 ± 1.3%), and OCR/DNA values (175.7 ± 15.4) were higher to those of human islets isolated at the University of Arizona (n=25, 136.5 ± 5.5) [Figure 1]. Glucose-stimulated dynamic perifusion demonstrated a robust biphasic insulin secretion that was suppressed under low-glucose conditions, with a stimulation index comparable to that of human islets [Figure 2]. hSC-islets also responded to cAMP amplification (exendin-4) with significantly enhanced insulin secretion compared to glucose stimulation alone.
Conclusion: These findings demonstrate that hSC-islets can be shipped internationally using silicone rubber membrane vessels while retaining high viability and functionality. This supports the potential use of hSC-islets as a promising alternative to human islets as well as the benefits of using gas-permeable vessels. Future work will include performing the same panel of assessments pre-shipment to enable direct comparisons as well as transplantation into diabetic rodents to demonstrate in vivo functionality.