
Madi began their scientific career with a BSc in Biomedical Science from Newcastle University, where a strong interest in immunology and translational medicine developed. This foundation led to a PhD in Transplant Science, focusing on the investigation of cellular therapy fate through advanced tracking in novel clinical models. Their doctoral work explored how cellular therapies behave and persist in vivo, contributing to a better understanding of therapeutic mechanisms and outcomes in transplantation.
Recently, Madi accepted a postdoctoral position in complement research, where they are involved in the evaluation of novel complement-targeted therapies. Their current work aims to bridge fundamental complement biology with translational applications in immune-mediated and inflammatory diseases.
Improved understanding of cell fate using a kidney normothermic machine perfusion model
Madison Cooper1,2, Samuel J Tingle1,2,3, Saimir Luli4, Mary A Neal1,5, Peter E Thelwall1,5, Marnie L Brown1,2, Chloe Connelly1, Beth Gibson1, George Kourounis1,2,3, Samantha Lee1,2, Rhys R Pook1,2, Mangala Srinivas6, Lucy Bates1,2, Colin Wilson1,2,3, Neil Sheerin1,2,7, William E Scott III1,2.
1Translational and Clinical Research Institute, Newcastle University, Newcastle upon Tyne, United Kingdom; 2NIHR Blood and Transplant Research Unit, Newcastle and Cambridge University, Newcastle upon Tyne, United Kingdom; 3Institute of Transplantation, Freeman Hospital, Newcastle upon Tyne, United Kingdom; 4Preclinical In Vivo Imaging, Newcastle University, Newcastle upon Tyne, United Kingdom; 5Newcastle University Newcastle Magnetic Resonance Centre, Newcastle University, Newcastle upon Tyne, United Kingdom; 6Department of Cell Biology and Immunology, Wageningen University & Research, Wageningen, Netherlands; 7Renal Services, Newcastle Upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, United Kingdom
A significant barrier to the clinical translation of cellular therapies is the limited understanding of cell fate and distribution following administration in humans. While numerous biodistribution studies exist in animal models, human data remains sparse due to the invasive nature, limited resolution and short tracking windows of current imaging modalities. To bridge this gap, novel preclinical platforms such as normothermic machine perfusion (NMP) are being developed. NMP models, which replicate human physiological conditions, enable the use of non-transplantable organs as experimental platforms to assess cell engraftment and behaviour. To support such studies, labelling protocols for multiple cell lines using the tracking agent 19F-nanoparticles (19F-NP) were optimised to establish detection thresholds and imaging sensitivity across various modalities. PMA differentiated THP-1 macrophages (THP-1m) demonstrated the highest 19F-NP uptake and detectability, justifying their selection for proof-of-concept NMP cell tracking experiments.
THP-1 monocytes were differentiated into THP-1m by treating with 100 ng/mL Phorbol 12-myristate 13-acetate (PMA) for 48 hrs at 37°C. The THP-1m cells were then incubated with 19F-NPs conjugated with a Nile Red fluorophore (20 mg/mL) in serum-free RPMI 1640 medium for 18 hrs. Following labelling, 5×10⁷ THP-1m cells were cryopreserved in liquid nitrogen. Subsequently, a research human kidney was perfused ex vivo 7 hrs with a blood-based perfusate at 37°C. Throughout the perfusion, biopsies, blood and gas samples, urine output, and Contrast-Enhanced Ultrasound (CEUS) images were collected. 1 hr into perfusion, the thawed, labelled THP-1m macrophages were delivered into the arterial tap of the circuit. At the final timepoint, the organ was analysed using In Vivo Imaging System (IVIS) and Magnetic Resonance Imaging (MRI) to investigate the distribution and localisation of the THP-1ms.
Increased signal intensity was observed in both contrast and B mode of the CEUS images, with strongest signal localised to the renal cortex. This was observed at all timepoints up to 7 hrs during perfusion. Post-perfusion, the remaining kidney tissue was bi-sected and scanned using IVIS revealing a bright fluorescent signal throughout the cortex of the bisected kidney and not the medulla. Nile Red signal was also observed within cortical glomeruli and around medullary vessel endothelium with immunofluorescent microscopy. Co-localisation of Nile Red with the CD14 macrophage marker confirmed the signal origin to be THP-1m infused cells (figure 1).
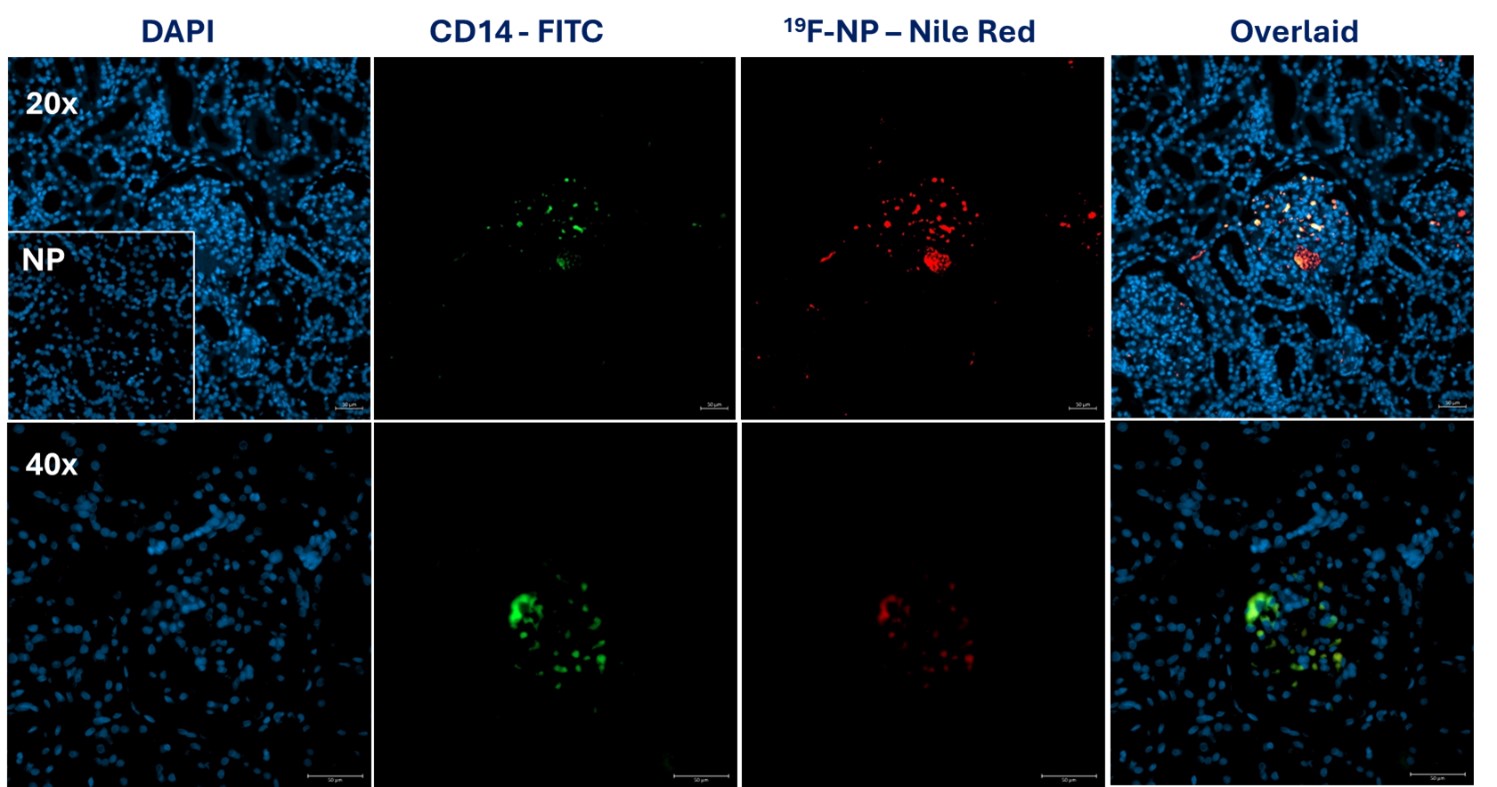
These findings demonstrate a proof of concept for the use of NMP as a human model system to track cell fate using multiple imaging modalities. The next phase will apply 19F-NP-labelled THP-1ms to a Multi-visceral Perfusion (MVP) model to determine whether cells can be tracked between organs over an extended perfusion period. It is hoped that this model can help streamline our understanding of cell fate avoiding the ethical and mechanistic issues associated with animal models, reducing risk to patients and unlocking the potential for cellular therapies.
This study is funded by the National Institute for Health and Care Research (NIHR) Blood and Transplant Research Unit in Organ Donation and Transplantation (NIHR203332), a partnership between NHS Blood and Transplant, University of Cambridge and Newcastle University. The views expressed are those of the author(s) and not necessarily those of the NIHR, NHS Blood and Transplant or the Department of Health and Social Care. . This study is funded by the Health and Environmental Science Institute (HESI). The ‘HESI’ is organised and operated as a non-profit, charitable and scientific organisation.. Special thanks to Professor Mangala Srinivas for providing us with the 19F-NP, Dr Mary Neal and Professor Pete Thelwall for their expertise and MRI scanning, and Dr Saimir Luli for IVIS training, scanning and advice. . I would like to extend our gratitude to the organ donors and their families. .
[1] Cell Therapy
[2] Cell Fate
[3] Imaging
[4] Normothermic Machine Perfusion
[5] Human
[6] Multimodal Imaging
[7] Novel Platforms