The nude rat is capable of mounting a strong fibrotic response against subcutaneously implanted, cell-free encapsulation devices
Kelly Pasowisty1, Amy C. Kelly1, Trisha M. Fabijanic1, Carola G. Davila1, Leah V. Steyn1, Hannah A. Hagen1, Jennifer P. Kitzmann1, Thomas Loudovaris1, Robert Johnson2, Charles W. Putnam1, Klearchos K. Papas1,2.
1Department of Surgery, University of Arizona, Tucson, AZ, United States; 2Procyon Technologies LLC, Tucson, AZ, United States
Introduction: Subcutaneous (SC) implantation of encapsulated allogeneic cells has gained considerable attention for therapeutic applications in humans. A key issue when introducing biomaterials is that they may induce a foreign body response (FBR), a critical obstacle to their clinical applications. The nude rat—a T-cell-deficient, B-cell-competent strain—is a valuable preclinical model for testing encapsulation devices containing human cells, as it can accept cellular xenografts without immunosuppression (IS). However, using nude rats in preclinical studies has been questioned due to concerns about their ability to generate an FBR. Nonetheless, a recent report describes a robust FBR in nude rats to 0.45µm porous, cell-impermeable PTFE inner membranes used in cell-containing encapsulation devices1. This study examined in detail the capability of the nude rat to mount fibrotic responses to cell-free biomaterials used in encapsulation devices.
Methods: Encapsulation devices composed of smooth plastics and small-pore immunoprotective membranes with or without vascularizing membranes were implanted in male Crl:NIH-Foxn1rnu athymic nude rats or Sprague-Dawley (SD) rats. Devices were explanted at 1 or 3 months, formalin-fixed, paraffin-embedded, sectioned, and analyzed histologically after hematoxylin and eosin (H&E) and Masson’s trichrome staining. Fibrotic capsule thickness and its surface area coverage in representative sections were quantified using BZ-Analyzer software. Data are reported as the Mean and Standard Error of the Mean (SEM). Statistical significance was assessed using one-way ANOVA.
Results: Nude rats exhibited robust fibrotic responses to 0.4µm immunoprotective membranes (i.e., Biopore) with a surrounding capsule thickness of 236µm (n = 6, SEM 25) at 1 month (Figure 1A), persisting at 3 months post-implant. The fibrosis-generating effect was observed across all animals of both strains and various smooth membranes, but not in devices with vascularizing membranes (pore size 5-10µm). The FBR noted in rats was comparable to that observed in sheep and domestic pigs with the same materials; hence, the nude rat model replicates fibrotic responses of expensive large animal models.
Conclusion: Nude rats, despite their immunodeficiency, exhibit marked fibrotic responses to 0.4µm pore, immunoprotective membranes and similar clinically relevant biomaterials. Thus, the nude rat is a useful, relatively inexpensive preclinical model for evaluating host responses to biomaterials, especially those used in cell encapsulation devices. The nude rat model is advantageous compared to large animal models (e.g., pigs, non-human primates, and sheep), which require potentially confounding IS when human (xenogeneic) cells are implanted. Incorporation of a vascularizing membrane is a critical modification of implantable devices, promoting neovascularization and significantly reducing FBR (Figure 1B), thereby supporting long-term function.
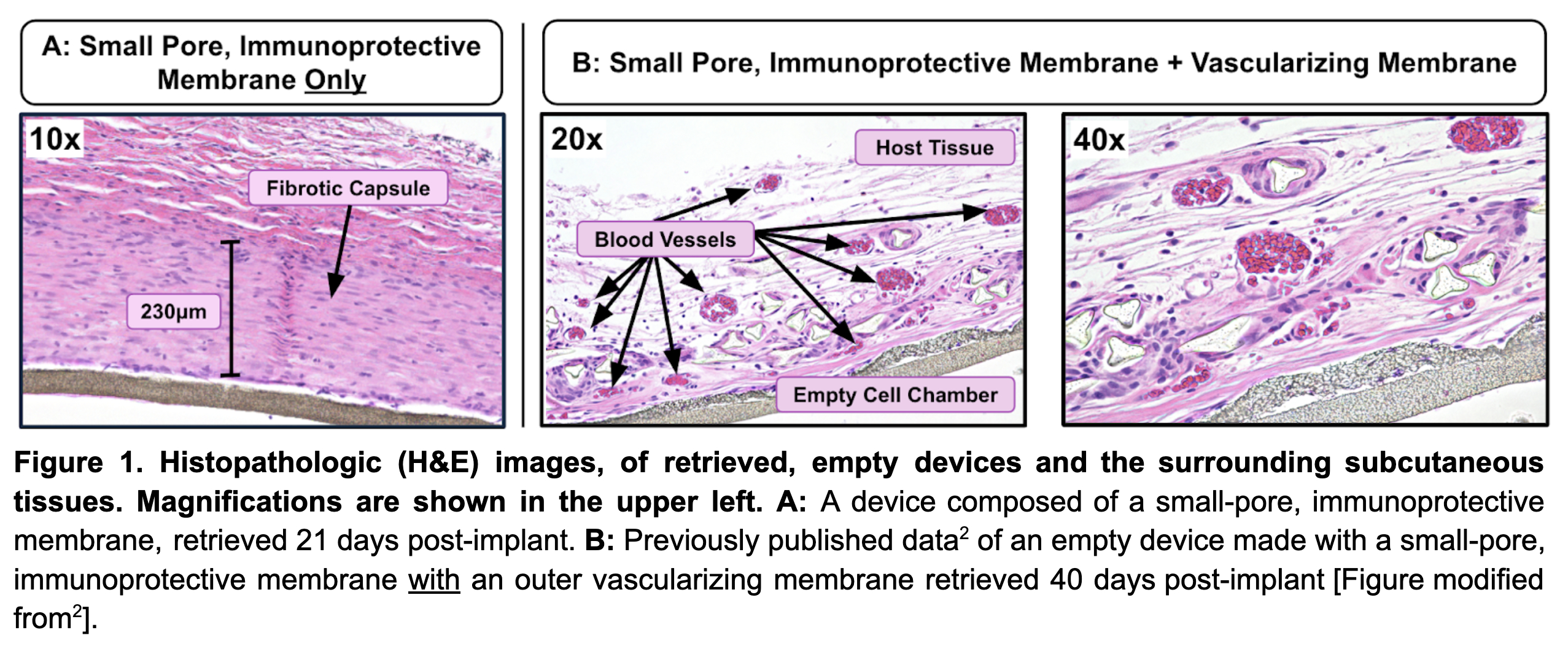
" href="https://app.ctrms2025.org/papers/body/303#">Add Figure 1
Nijns, J.R., De Mesmaeker, I., Suenens, K.G., Stangé, G.M., De Groot, K., Marques de Lima, M., Kraus, M.R.C., Keymeulen, B., Waelput, W., Jacobs-Tulleneers Thevissen, D., and Pipeleers, D.G. (2023). Comparison of Omentum and Subcutis as Implant Sites for Device-Encapsulated Human iPSC-Derived Pancreatic Endoderm in Nude Rats. Cell Transplant 32, 9636897231167323. 10.1177/09636897231167323.. Steyn, L.V., Drew, D., Vlachos, D., Huey, B., Cocchi, K., Price, N.D., Johnson, R., Putnam, C.W., and Papas, K.K. (2023). Accelerated absorption of regular insulin administered via a vascularizing permeable microchamber implanted subcutaneously in diabetic Rattus norvegicus. PLoS One 18, e0278794. 10.1371/journal.pone.0278794..
[1] Vascularizing membrane
[2] Foreign body response (FBR)
[3] Encapsulation device
[4] Immunoprotective membrane
[5] Implanted biomaterials
[6] Nude rat (Crl:NIH-Foxn1rnu)
[7] Cell encapsulation