
Autologous transplantation of LCAT gene-transduced adipocytes enabled long-term LCAT replacement in a patient with LCAT deficiency in first in human clinical trial
Masayuki Kuroda1, Jun Wada2, Yoshiro Masezawa3, Yoshitaka Kubota4, Nobuyuki Mitsukawa4, Kunio Yasunaga5, Tokuo Yamamoto5, Masayuki Aso5, Hideki Hanaoka6, Yasushi Saito7, Koutaro Yokote3,7.
1Center for Advanced Medicine, Chiba University Hospital, Chiba, Japan; 2Department of Nephrology, Rheumatology, Endocrinology and Metabolism, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan; 3Department of Endocrinology, Hematology and Gerontology, Chiba University Graduate School of Medicine, Chiba, Japan; 4Department of Plastic Surgery, Chiba University Graduate School of Medicine, Chiba, Japan; 5CellGenTech, Inc., Chiba, Japan; 6Clinical Research Center, Chiba University Hospital, Chiba, Japan; 7Chiba University, Chiba, Japan
Introduction: Familial lecithin:cholesterol acyltransferase (LCAT) deficiency (FLD) syndrome is an autosomal recessive disease characterized by low plasma LCAT activity and severe dysfunction of HDL with subsequent impaired lipoprotein metabolisms. Patients often develop complications such as corneal opacity, hemolytic anemia, and renal dysfunction. In plasma protein deficiency such as LCAT deficiency, gene therapy-mediated continuous enzyme replacement is the most suitable therapeutic approach. We have postulated adipocyte-based ex vivo gene therapy-mediated enzyme replacement therapy (ERT), in which genetically-modified adipocytes (GMAC) reside at transplanted sites to continuously supply therapeutic proteins. We have applied our GMAC technology to treat FLD via transplantation of LCAT gene-transduced autologous preadipocytes (LCAT-GMAC).
Methods: The ex vivo gene therapy protocol has been approved under the Act on Securement of Safety of Regenerative Medicine in Japan. A patient was enrolled in January of 2017, and LCAT-GMAC was administered subcutaneously with fibrin glue, which is a clinically applicable scaffold, and the clinical evaluation of the prescribed observation and subsequent follow-up period (total of 240 weeks after administration) was completed, then safety as well as efficacy of the LCAT-GMAC has been investigated in the patient.
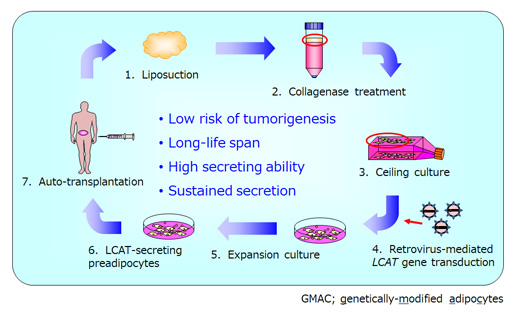
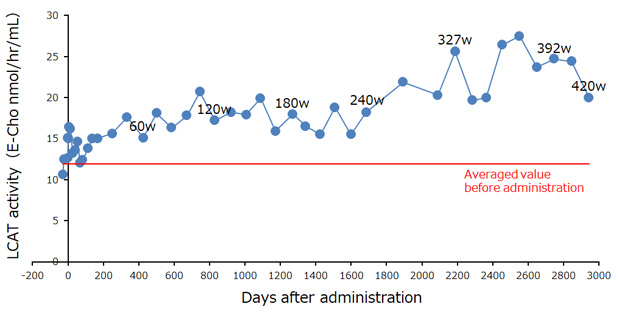
Results: Post administration pain was observed, however, no other treatment-related adverse effects were observed. In addition, no replication-competent retrovirus in patient's blood sample or tumorigenicity of the LCAT-GMAC was observed. Increased serum LCAT activity has sustained for 240 weeks. Together with changes in laboratory values related to lipid metabolism, the long-term amelioration of abnormal lipoprotein metabolism was suggested in the patient. The hemoglobin-haptoglobin complex produced with hemolytic anemia was also reduced, which suggested improved vulnerability of erythrocytes. The patient is still undergoing follow-up and still has persistent LCAT activity in his blood eight years after the administration of LCAT-GMAC.
Conclusion: These results suggest that LCAT-GMAC is a curative ex vivo gene/cell therapy product for FLD patients, and the following multicenter clinical trials are currently underway to obtain regulatory approval. In conclusion, the GMAC technology will provide a novel platform for ex vivo gene therapy mediated ERT for a variety of intractable diseases.
[1] adipocytes
[2] ceiling culture
[3] ex vivo gene/cell therapy
[4] enzyme replacement therapy
[5] Familial lecithin:cholesterol acyltransferase deficiency