
Oxygen enabled, high capacity, open encapsulation devices implanted subcutaneously vascularized and reversed diabetes in immunodeficient rats
Amy Kelly1, Trisha M Fabijanic1, Jennifer P Kitzmann1, Carola G Davila1, Delaney A Drew1, Craig Weber1, Ronald M Lynch2, Robert Johnson3, Thomas Loudovaris1, Charles W Putnam1, Klearchos K Papas1,3.
1Surgery, University of Arizona, Tucson, AZ, United States; 2Physiological Sciences, University of Arizona, Tucson, AZ, United States; 3Procyon Technologies LLC, Tucson, AZ, United States
Introduction: The ambient pO2 of subcutaneous (SC) tissue is insufficient to support the functionality of human islets in encapsulation devices until sufficient neovascularization has occurred. The vascularizing oxygen-enabled, non-immunoisolating encapsulation device (ONID) was created to support the high densities of islets required for reversal of clinical diabetes. Here, we report that human islets loaded at high density in ONIDs having an O2 supply immediately restore normoglycemia; islet cell function is sustained for the ~6 weeks required to develop a robust vasculature about the device. Then, discontinuing the O2 supply does not compromise islet functionality.
Methods: Male Crl:NIH-Foxn1rnu nude rats rendered diabetic with streptozotocin were implanted with a single oxygenated device (ONID) consisting of an oxygenated lumen bordered by two islet-containing chamber the outer membranes of which facilitate vascularization of the device (Fig. 1).
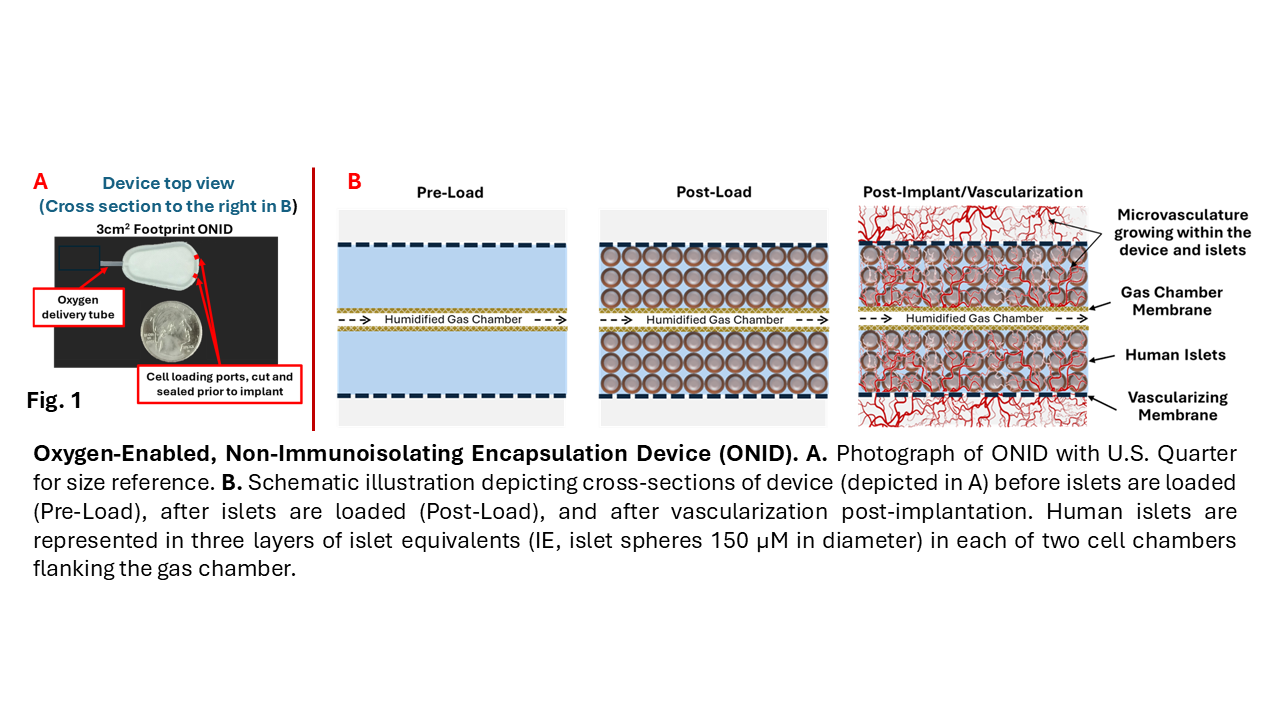
O2 gas is continuously delivered to the oxygen chamber via a transdermal tube. Control diabetic rats were implanted with empty ONIDs. Blood glucose concentrations were measured daily. Vascularization of the ONID was evaluated in vivo by computerized tomography (microCT). Explanted ONIDs were examined histologically after H&E staining, and IHC for CD3, Ku80 and insulin. Enumeration of close vascular structures (CVS: blood vessels within 15µm of the inner membrane) was performed manually and normalized to membrane area.
Results: Diabetic nude rats (n=10) transplanted with ONIDs + human islets had significantly lower blood glucose concentrations than both their pre-transplant values and the control subjects. Normoglycemia was frequently attained immediately after ONID implantation and persisted for the 12-week study. If O2 delivery was inadvertently disrupted within the first 2 weeks, blood glucose values soon increased; thus the O2 supply was essential to islet function. In contrast, normoglycemia was not perturbed when O2 delivery was purposely discontinued at 6 weeks and persisted until ONID explantation. Histologically, host SC tissue surrounding the ONID was devoid of fibrosis and exhibited a robust vasculature penetrating the cell chamber. CVS were similar to values reported for the native pancreas and with other SC encapsulation technologies (Fig. 2).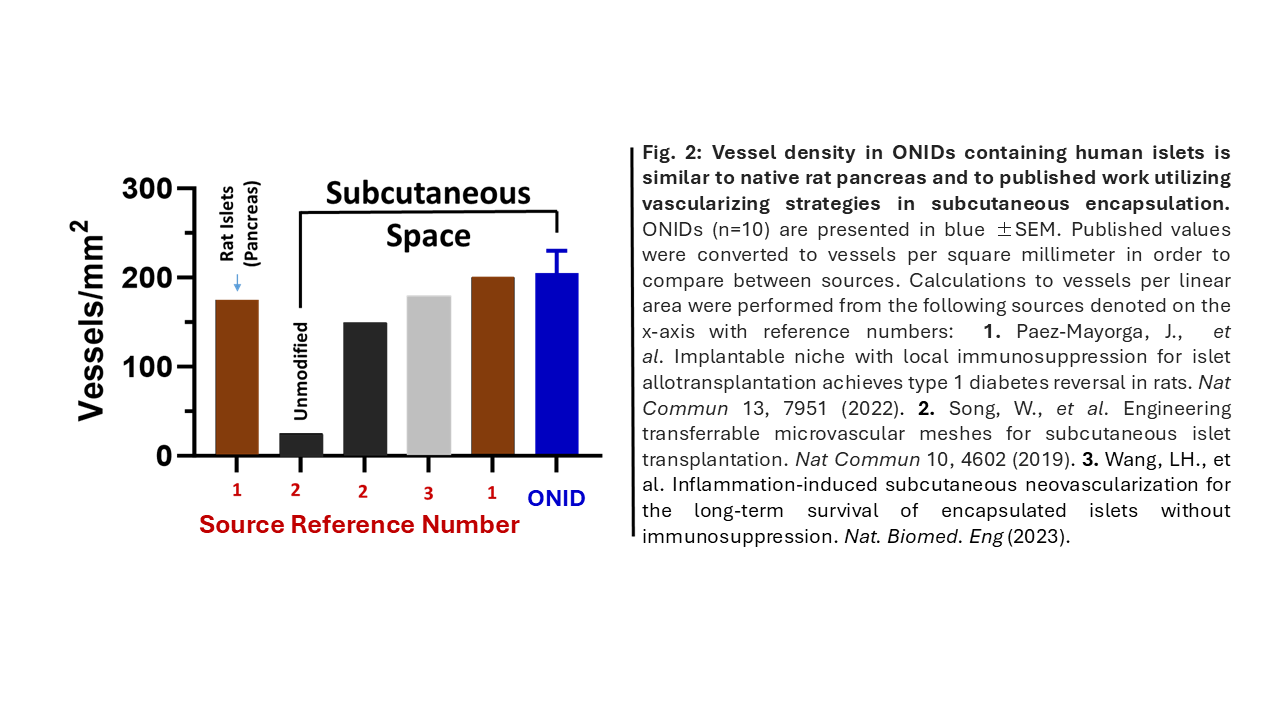
Conclusion: Oxygen-supplemented, SC implanted ONIDs containing high densities of human islets reversed diabetes in nude rats. Vascularization surrounding the ONID correlated with tolerance to discontinuation of O2. Importantly, the 3cm2 footprint ONID supported 80,000 IE human islets, a clinically relevant density. This proof-of concept study with human islet-containing ONIDs reaffirms the essentiality of supplemental oxygen until a supportive vasculature can provide sufficient O2.
Breakthrough T1D 3-SRA-2023-1437-M-B.