
Amnion epithelial cell secretome profile achieved by an optimized proteomic workflow
Nicole Grinovero1, Alessia Gallo2, Vitale Miceli2, Andrea Petretto1, Roberto Gramignoli3,4.
1Core Facility for Omics Sciences, IRCCS Istituto Giannina Gaslini, Genoa, Italy; 2Research Department, IRCCS ISMETT, Palermo, Italy; 3UOSD for Cell Therapy Lab, IRCCS Istituto Giannina Gaslini, Genova, Italy; 4Department of Laboratory Medicine, Karolinska Institutet, Stockholm, Sweden
Introduction: Multipotent human Amnion Epithelial Cells (hAECs) are gaining recognition as a novel therapeutic strategy. Safety and efficacy in reversing congenital defects or chronic disorders are not always coupled with maturation into a tissue-specific phenotype. hAECs offer paracrine support to restore architecture and function to damaged organs. Thus, we performed a complete profiling of hAEC secretome, essential to promote such advanced medicinal products to the clinic, and to match patients’ needs. However, secretome profiling is challenged by high dynamic range in protein concentration, interference from culture supplements, and significant inter-donor and condition-dependent variability. To address these limitations, we implemented a robust, high-throughput mass spectrometry-based proteomics workflow for the untargeted analysis of hAEC-conditioned media across diverse experimental conditions, including variations in donor origin, media supplementation, oxygen levels, and pro-inflammatory priming.
Methods: hAEC from 20 donors were isolated according to cGMP procedures, and validated both on intact cells and released vesicles (hAEV). We exposed hAEC to different culture conditions and stimuli, aimed to enhance their secretory properties. Conditioned media from 96 samples were processed using a semi-automated workflow to isolate proteins via Protein Aggregation Capture (PAC) on magnetic beads [1]. Following enzymatic digestion, peptides were purified using an in-StageTip method [2] and analysed by data-independent acquisition on a high-resolution Orbitrap mass spectrometer.
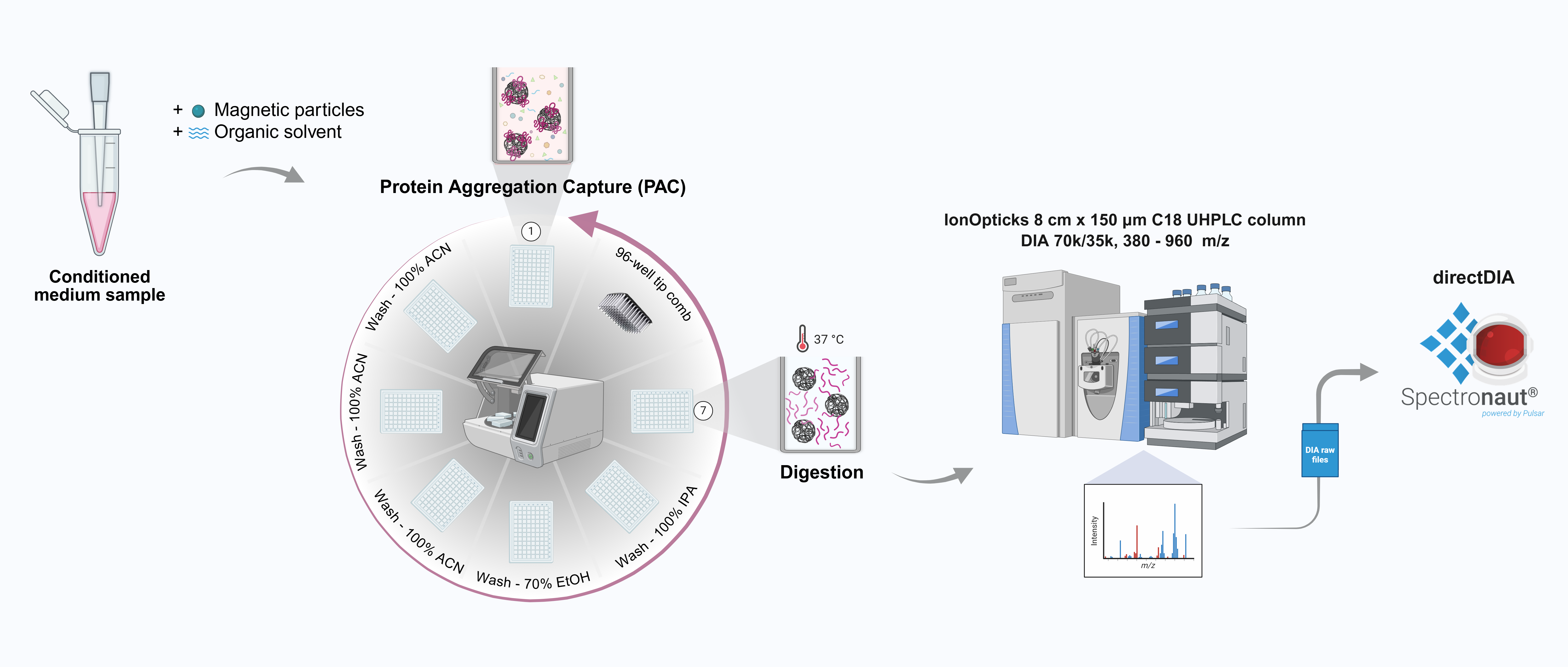
Results: We identified several mediators and enzymes on the surface of intact hAEC, transferred to hAEVs. Normoxic and hypoxic conditions resulted in minor variability in solutes (>80% preserved; differences in VEGF, IL1b, IL6, IL8, and CxCL1). Robust and constitutive secretion of immunomodulatory molecules, such as HLA-G and PD-L1, was measured within the first days of culture, and maintained in presence of inflammatory stimuli. Preliminary data confirm that the workflow ensures good peptide recovery and consistent signal across different experimental conditions. A complete profile analysis will reveal common versus donor-specific proteins, in relation to external stimuli.
Conclusions: The ability to treat most common (chronic/congenital) diseases with allogeneic stem cells without matching or immunosuppressive drugs may represent a “game changer” for several patients (pediatric first), frequently orphans from treatment. We validated and optimized a scalable and reproducible workflow enabling large-scale secretome profiling, with high sensitivity but low sample input. Such a technology is ideal for large-cohort studies and validation of innovative applications, such as hAEC-derived soluble mediators.
References
[1] Amnion Epithelial Cells
[2] Proteomics
[3] Secretome