
Dr. Hirotake Komatsu, Associate Professor at the Department of Surgery at UCSF, is at the forefront of diabetes treatment through beta cell replacement therapy. With extensive clinical experience as a surgeon and a Ph.D. in Molecular Biology, his research focuses on the assessment of the hypoxia response in pancreatic islets, minimally invasive transplantation techniques, and improving cell survival with innovative oxygenation approaches. His contributions include developing an implantable oxygenation device for improving extrahepatic site islet transplantation outcomes. Recognized for his work with several grants and publications, Dr. Komatsu's expertise is also evidenced by his active role in academic and professional communities, including serving on editorial boards and as a young investigator committee member of the International Pancreas and Islet Transplantation Association. His collaborative projects bridge islet physiology and engineering fields, aiming to revolutionize the treatment of diabetes and enhance islet engraftment.
Oxygen-releasing coaxial nanofiber membrane for islet cell transplantation to address hypoxia-induced graft death
Hiroyuki Kato1, Brooke Campbell2, Houra Mobaleghaleslam2, Robert W Horvath2, Mona Sheta1, Hiroaki Mitsugashira1, Jingjing Yang3, Katsuhiro Esashika3, Shunya Mizuno3, Daewoo Han2, Andrew Steckl2, Hirotake Komatsu1.
1Surgery, University of California, San Francisco, San Francisco, CA, United States; 2Electrical Engineering and Computer Science, University of Cincinnati, Cincinnati, OH, United States; 3Mitsui Chemicals, Inc, Tokyo, Japan
Introduction: Beta cell replacement via islet transplantation offers a potential cure for insulin-dependent diabetes. However, engraftment efficiency of transplanted islet cells remains suboptimal across all implantation sites, including current clinical (intrahepatic) site and alternative extrahepatic locations. Unlike organ transplants, islet grafts inherently face acute hypoxia before vascularization. We address this by developing a transplantable oxygen (O₂)-releasing nanofiber membrane for extrahepatic use.
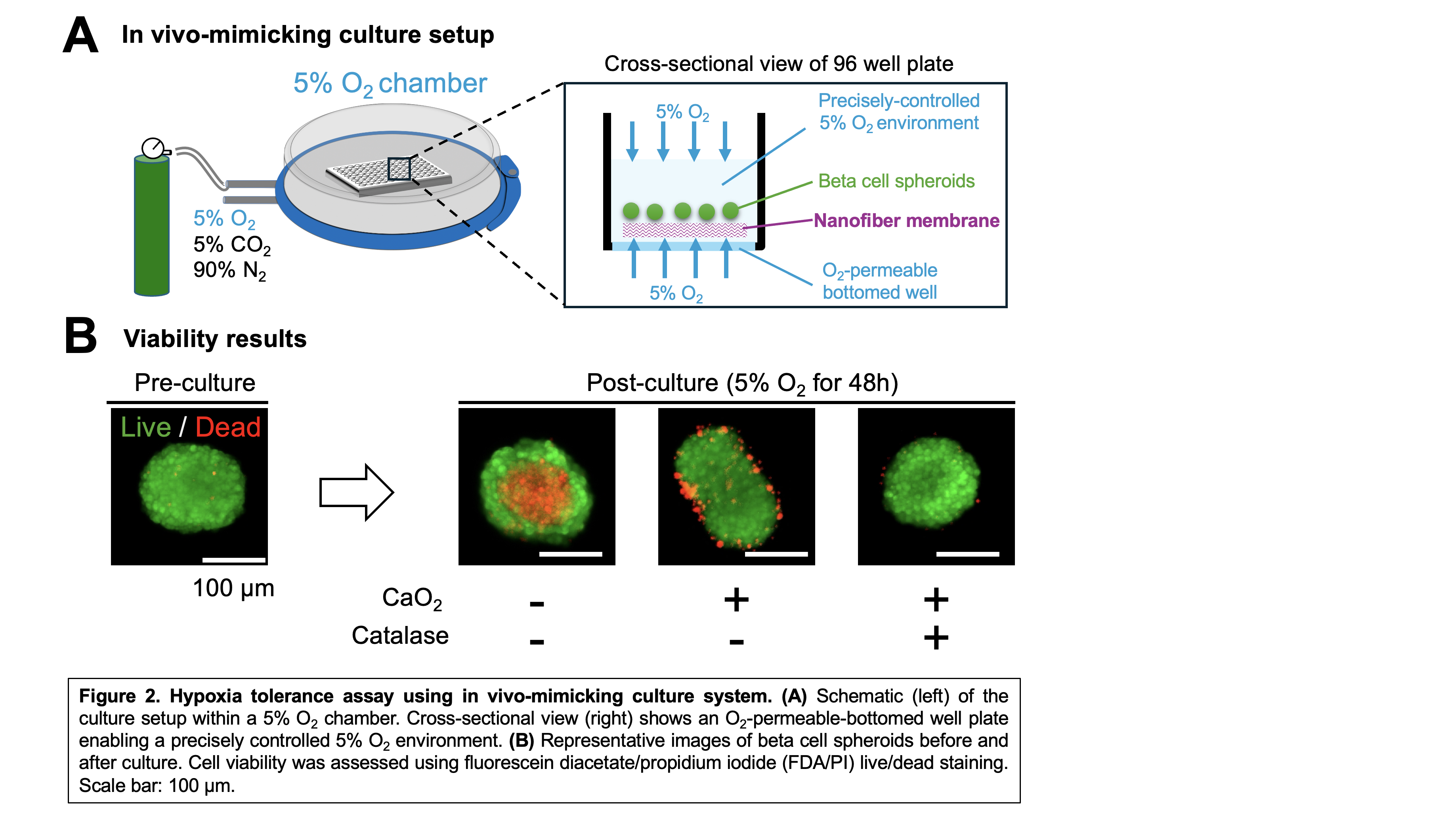
Methods: Electrospun nanofiber membranes were fabricated as single-fiber or coaxial-fiber types (Fig. 1A), including: (1) native (nonloaded) nanofibers; (2) calcium peroxide (CaO₂)-loaded nanofibers; and (3) CaO₂ plus catalase-loaded coaxial nanofibers. CaO₂, embedded in a polycaprolactone/polyvinylpyrrolidone (75:25) polymer matrix, served as the O₂ source. In coaxial fibers, catalase was incorporated into the polyethylene oxide sheath to enhance O₂ release and reduce the accumulation of cytotoxic hydrogen peroxide (H₂O₂). Membranes were 100–150 µm thick. Biocompatibility was tested by implanting 7 × 7 mm native membranes into the subcutaneous space of Lewis rats, followed by histology. O₂ release was measured over 7 days using 1 × 1 cm sheets in PBS, with daily O₂ readings. For in vitro testing, 0.25% CaO₂ nanofibers were used to culture ~100 INS-1-derived beta cell spheroids (150–200 µm) under 5% O₂ for 2 days. Viability was assessed with fluorescein diacetate/propidium iodide live/dead staining.
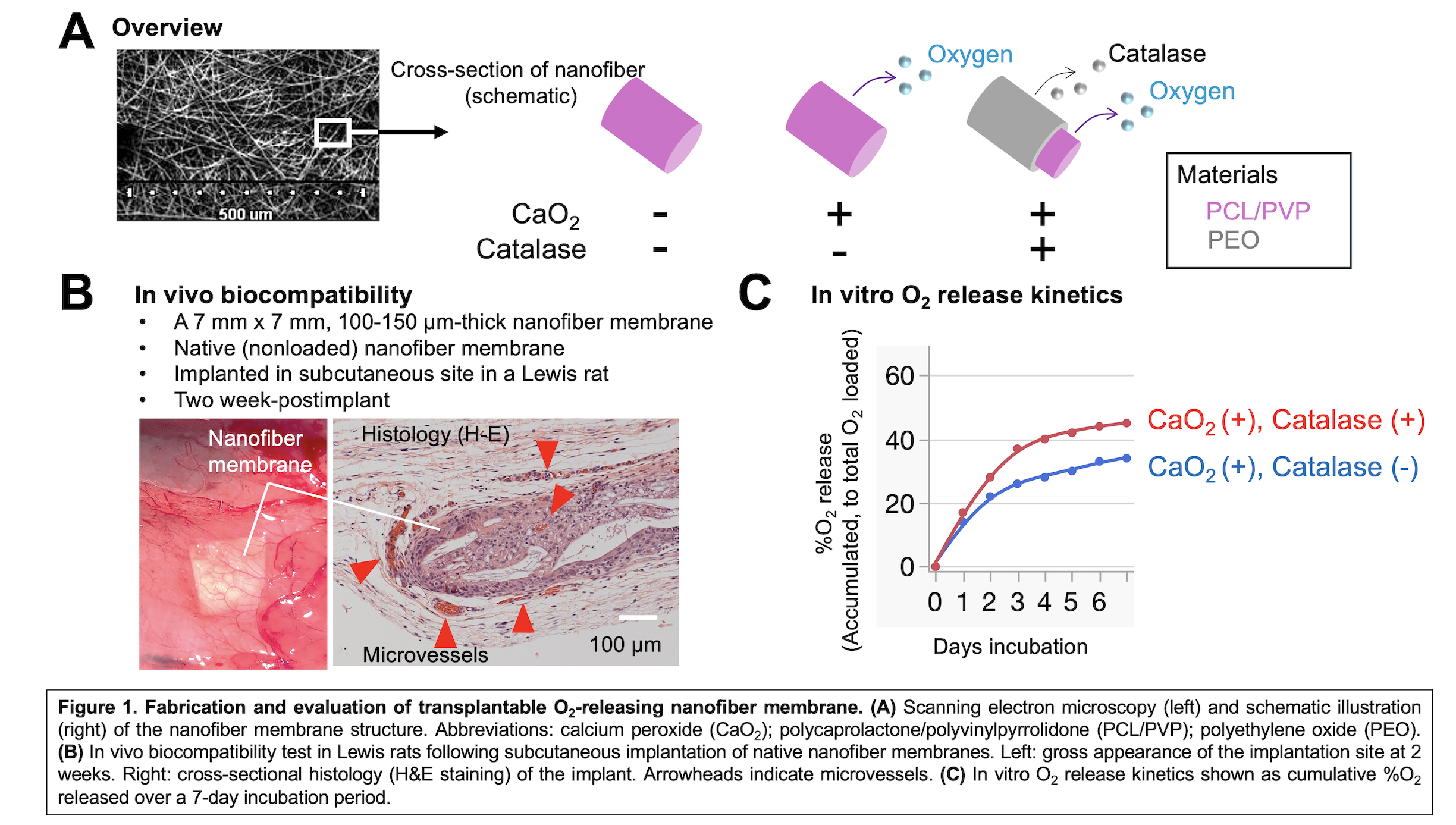
Results: Histological analysis at two weeks post-implantation confirmed that the nanofiber membranes were biocompatible. Host-derived angiogenesis was observed around and within the porous nanofiber structure (Fig. 1B). In vitro O₂ release analysis showed that catalase-containing coaxial nanofibers significantly enhanced O₂ delivery compared to nanofibers containing only CaO₂, especially within the first four days (Fig. 1C). In the hypoxia tolerance test, O₂-permeable-bottom 96-well plates (InnoCell™, Mitsui Chemicals, Inc.) and a hypoxia chamber enabled uniform and controlled 5% O₂ environment with minimal gradients within the culture medium (Fig. 2A). Beta cell spheroids cultured on native nanofibers exhibited prominent central necrosis (Fig. 2B). This necrotic core was alleviated in spheroids cultured on CaO₂-only nanofibers, although surface damage was observed. Importantly, addition of catalase in coaxial nanofibers prevented surface damage and central necrosis, indicating that catalase effectively mitigated cytotoxic H₂O₂ accumulation while providing sufficient oxygenation for beta cell survival.
Conclusion: Coaxial nanofiber structures enable the co-delivery of CaO₂ and catalase, enhancing oxygenation while reducing cytotoxic effects. This approach represents a promising strategy for overcoming hypoxia-induced graft failure in islet cell transplantation, particularly in extrahepatic sites.
Breakthrough T1D (formerly Juvenile Diabetes Research Foundation), Grant Number: 3-SRA-2023-1425-S-B. Nora Eccles Treadwell Foundation (no grant number).
[1] Beta cell replacement
[2] Diabetes
[3] Coaxial Nanofiber
[4] Oxygen Release Scaffold
[5] Calcium Peroxide
[6] Hypoxia
[7] Islet Transplantation
[8] In vivo-mimicking culture
[9] O₂-Permeable Plate