First-in-human xenotransfusion of genetically engineered pig red blood cells: A brain-dead human model
Tao Li1, Hidetaka Hara1, Hongtao Jiang1, Huiling Gan1, Ting Yan1, Jianan Zhang1, Yong Wang2, Hang Yu1, Dengke Pan2, Yi Wang1.
1The Transplantation Institute of the Second Affiliated Hospital, Hainan Medical University, Haikou, People's Republic of China; 2Chengdu Clonorgan Biotechnology Co., Ltd, Chengdu, People's Republic of China
Xenotransplant Research Group.
Introduction: Xenotransfusion using genetically engineered (GE) pig red blood cells (pRBCs) represents a promising approach to address critical blood shortages, particularly in emergency settings. Although nonhuman primates (NHPs) are widely used in preclinical studies, their species-specific immune responses limit translational relevance, highlighting the need for alternative models that more closely approximate human immunology. This study aimed to evaluate the suitability of brain-dead human subjects as a preclinical model for xenotransfusion by comparing their hematological and immunological profiles with those of acute blood loss (ABL) patients and assessing the feasibility, safety, and immunological responses to GE pRBCs in a brain-dead human subject.
Methods: Comprehensive evaluations of hematologic, biochemical, coagulation, and immunologic parameters were performed in brain-dead donors (n=179) and ABL patients (n=104) requiring transfusion. In vitro assessments of anti-triple-knockout (TKO) pig RBC responses were also performed in brain-dead subjects (n=31) and compared to those of ABL patients (n=102). RBCs from TKO pigs expressing human CD55 (housed under pathogen-free conditions) were labeled with CFSE for tracking. A single brain-dead human subject received a transfusion of GE pRBCs, with pre-transfusion treatments including an anti-C5 inhibitor, corticosteroids, and antihistamines. Monitoring included continuous vital sign tracking, hematological and immunological assessments, complement activation assays, and pathogen screening.
Results: Brain-dead subjects exhibited clinical and immunological profiles comparable to ABL patients. IgM and IgG binding levels to TKO RBCs and CDC activity were not significantly different between groups, supporting the model’s clinical relevance. In the transfused subject, GE pRBCs exhibited a biphasic clearance pattern, with 50% of transfused cells remaining at 24 hours, 15% at 48 hours, and complete clearance by 72 hours. Pre-transfusion anti-pig IgM levels were low, transiently decreased post-transfusion, and rebounded by day 3. Anti-pig IgG levels, initially undetectable, significantly increased by day 7, accompanied by heightened complement-dependent cytotoxicity and hemagglutination. Both classical and alternative complement pathways were activated but moderated by regulatory mechanisms. No pig-derived pathogens were detected.
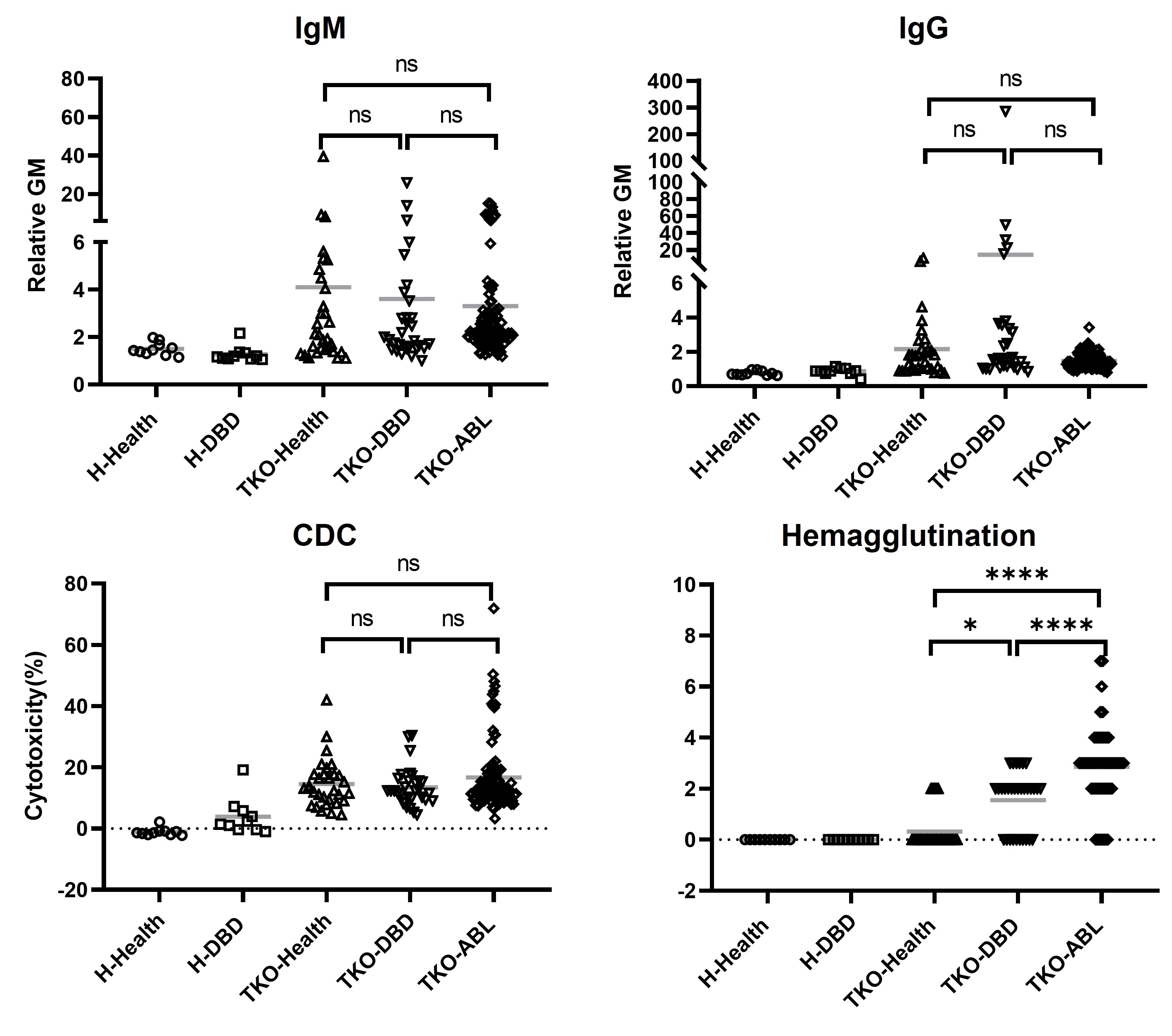
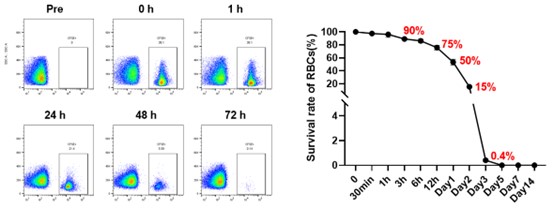
Conclusion: Brain-dead human subjects closely replicate the clinical conditions of ABL, providing a valuable preclinical model for xenotransfusion research, particularly in settings where NHP models have limitations. This study establishes the feasibility and safety of GE pRBC xenotransfusion, offering critical insights into immune responses and pathogen safety. The absence of zoonotic transmission and the manageable immune profile observed in this first-in-human trial underscore the potential of xenotransfusion as a promising solution to global blood shortages in critical care.
This study was supported in part by the National Key Research and Development Program (2023YFC3404304: Y.W., 2024YFC3406800: H.J.), National Natural Science Foundation of China (82260154: Y.W., 82460153: H.J., 82400891: T.L.), Hainan Provincial Science and Technology Talent Innovation Project (Category B) (KJRC2023B08: Y.W.), and the Academic Enhancement Support Program of Hainan Medical University (XSTS2025029: H.H., XSTS2025161: T.L). H.H. is also supported by the Hainan Provincial High-Level Foreign Experts Recruitment Program (G20250218019E)..
[1] xenotransfusion
[2] genetically engineered pig
[3] brain-dead human model
[4] acute blood loss
[5] immune responses