Development of dutasteride loaded dissolving microneedles for transdermal permeation
Chul hyun Park1, Ga-Young Sim1, Minseok Kang1, Nahyun Kim1, Youn seop Kim1, Dongho Lee1, Kyungho Baek Ph.D1,2, Han-Gon Choi Ph.D2, Sung Giu Jin Ph.D1.
1Department of Pharmaceutical Engineering, Dankook University, Cheonan-si, Korea; 2Pharmacy & Insitute of Pharmaceutical Science and Technology, Hanyang University, Ansan-si, Korea
Introduction: Androgenic alopecia (AGA) is a chronic disease caused by hair follicle micronization by dihydrotestosterone (DHT), which affects more than 50% of the global adult population. Dutasteride is a drug that blocks the production of DHT by simultaneously inhibiting 5α-reductase types I and II, and is widely used to treat prostate hypertrophy (BPH) and AGA. However, dutasteride has low solubility (0.038 ng/mL). DMN systems are emerging as advanced alternatives that load drugs into polymer matrices, penetrate the epidermal layer (50-200 μm), and gradually dissolve in the body to provide the benefits of continuous drug release, ease of application, and painlessness. In this study, we intend to design a DMN system using the polymer Kollicoat®MAE 100P to enhance the transdermal delivery efficiency of dutasteride.
Methods: DMN were fabricated using a solvent casting method using a vacuum. The first casting solution loading dutasteride (DTS), 10% w/v of Kollicoat®MAE 100P was dissolved in a water-ethanol combination (30% water and 70% ethanol by mass). A second casting solution for the backing layer was fabricated by dissolving PVP and PVA in water. Microneedle preparations were visualized using a digital microscope. The in vitro release profile was studied in distilled water and PBS pH 6.8, and studies of skin residual amount of DTS were performed using Franz diffusion cells in rat skin (PBS pH 6.8, 32 °C).
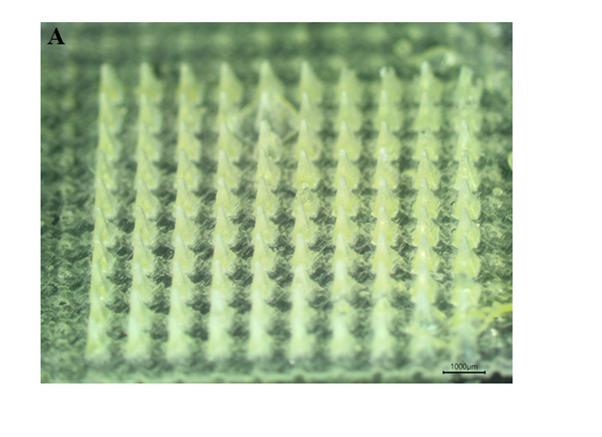
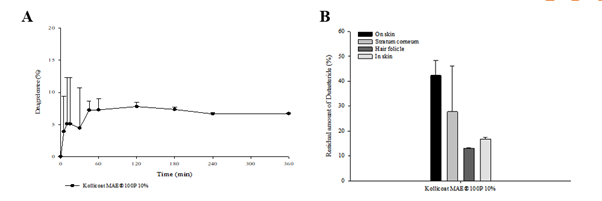
Results: Dutasteride-DMN could form a homogeneous microneedle array (Figure 1A). In vitro release studies showed that 7% of dutasteride remained after 1 h in PBS 6.8 (Figure. 2A). In an in vitro skin residual rate test, it was found that the residual amount in the follicles was 13.05 ± 0.1771%. (Figure 2B).
Conclusion: This study developed dutasteride-loaded DMNs designed to effectively penetrate the epidermis and deliver the drug directly to the dermis, thereby enhancing targeted therapeutic delivery. The DMNs were fabricated using a polymer matrix that improved the solubility of dutasteride, which is otherwise poorly soluble. Through in vitro studies, the DMNs demonstrated efficient drug release and significant accumulation in hair follicles, supporting their potential as an advanced treatment option for androgenic alopecia (AGA). Furthermore, these findings highlight the innovative applicability of DMN technology in transdermal drug delivery systems, suggesting that this approach could be adapted and further developed for a wide range of therapeutic areas beyond AGA, where targeted, painless, and efficient transdermal delivery is desirable.
References:
[1] Dutasteride
[2] Kollicoat MAE®100P
[3] Dissolving microneedles
Lectures by Chul hyun Park
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Thu-23 18:30 - 19:30 |
Poster Session | Development of dutasteride loaded dissolving microneedles for transdermal permeation | Hall A2-A4 |
