Enhancing immune cell therapy in hepatocellular carcinoma via pretreatment with all-trans retinoic acid
Massoud Vosough2,7, Neda Minaei1,2, Shokoufeh Torabi2,3, Marzieh Ebrahimi2, Ali Zarrabi4, Abbas Piryaei5,6.
1Department of Applied Cell Sciences, Royan Institute, Tehran, Iran; 2Department of Regenerative Medicine, Royan Institute, Tehran, Iran; 3Department of Tissue Engineering and Regenerative Medicine, Mazandaran University of Medical Sciences, Sari, Iran; 4Department of Biomedical Engineering, Istinye University, Istanbul, Turkey; 5Department of Biology and Anatomical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 6Urogenital Stem Cell Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 7Experimental Cancer Medicine, Karolinska Institutet and Karolinska University Hospital-Huddinge, Huddinge, Sweden
Hepatocellular carcinoma (HCC) remains a major cause of cancer-related mortality worldwide, with limited therapeutic success in advanced stages. Immune cell therapy utilizing cytokine-induced killer (CIK) cells has emerged as a promising strategy due to its MHC-unrestricted cytotoxicity and favorable safety profile.
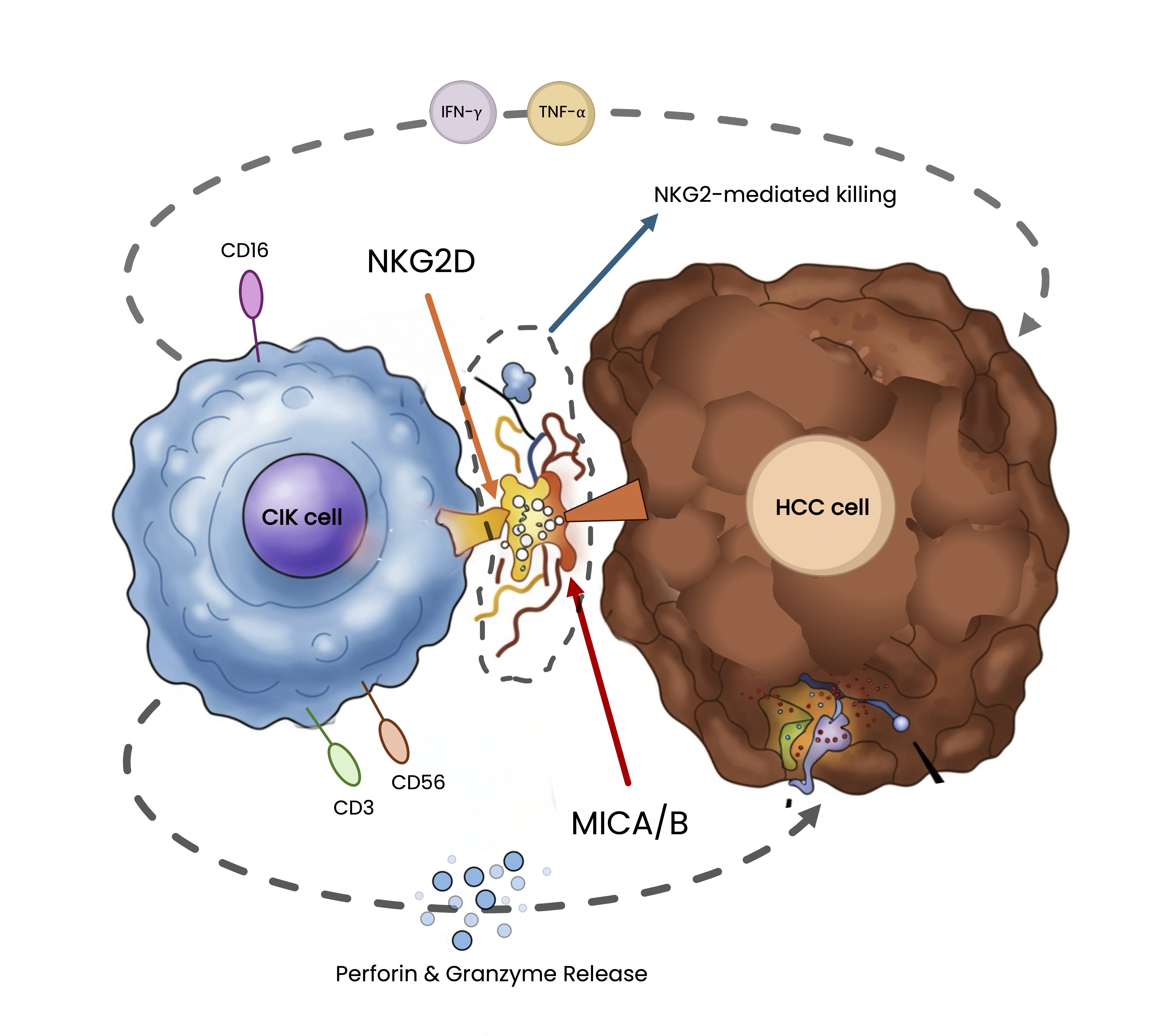
However, enhancing the responsiveness of tumor cells to immune effectors remains a clinical challenge. All-trans retinoic acid (ATRA), a derivative of vitamin A, has demonstrated the ability to modulate tumor cell phenotypes and induce expression of stress ligands which are key elements in immune recognition.
This study explores whether ATRA can sensitize HCC cells to immune-mediated cytotoxicity by upregulating surface molecules recognized by CIK cells. Using a human HCC cell line (HepG2), we assessed the impact of ATRA pretreatment on cellular proliferation, surface ligand expression, and susceptibility to CIK cell-mediated lysis. The functional activity of expanded CIK cells was evaluated in vitro and further tested in a subcutaneous xenograft model in immunodeficient mice.
Initial findings suggest that ATRA pretreatment reduces HCC cell proliferation and enhances immunogenic features associated with improved recognition by CIK cells. Co-culture experiments revealed synergistic effects on tumor cell apoptosis and cell cycle arrest. In vivo application of the combined approach indicated reduction in tumor volume and evidence of immune cell infiltration within the tumor site.
These results highlight the potential of pharmacological priming strategies, such as ATRA treatment, to enhance the therapeutic efficacy of CIK-based immune cell therapy. By promoting immune susceptibility of tumor cells, this approach may offer a complementary modality to improve clinical outcomes in HCC. Further mechanistic studies and translational assessments are warranted to refine this combination therapy for future application.
This study was funded by grants from Royan Lotus Charity Foundation (R1399- 99000074), Council for Development of Regenerative Medicine and Stem Cells Technologies, and Bahar Tashkhis Teb Co (BT-9906)..
References:
[1] Immunotherapy
[2] All-trans retinoic acid
[3] Cytokine-induced killer cells
[4] Hepatocellular carcinoma
[5] Differentiation therapy
[6] Tumor sensitization
Lectures by Massoud Vosough
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Thu-23 18:30 - 19:30 |
Poster Session | Enhancing immune cell therapy in hepatocellular carcinoma via pretreatment with all-trans retinoic acid |
